Whole chromosome aneuploidy in the brain of Bub1bH/H and Ercc1-/Δ7 mice
- PMID: 26681803
- PMCID: PMC4743693
- DOI: 10.1093/hmg/ddv612
Whole chromosome aneuploidy in the brain of Bub1bH/H and Ercc1-/Δ7 mice
Abstract
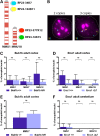
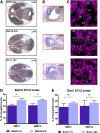
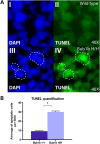
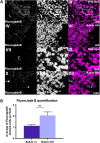
High levels of aneuploidy have been observed in disease-free tissues, including post-mitotic tissues such as the brain. Using a quantitative interphase-fluorescence in situ hybridization approach, we previously reported a chromosome-specific, age-related increase in aneuploidy in the mouse cerebral cortex. Increased aneuploidy has been associated with defects in DNA repair and the spindle assembly checkpoint, which in turn can lead to premature aging. Here, we quantified the frequency of aneuploidy of three autosomes in the cerebral cortex and cerebellum of adult and developing brain of Bub1b(H/H) mice, which have a faulty mitotic checkpoint, and Ercc1(-/Δ7) mice, defective in nucleotide excision repair and inter-strand cross-link repair. Surprisingly, the level of aneuploidy in the brain of these murine models of accelerated aging remains as low as in the young adult brains from control animals, i.e. <1% in the cerebral cortex and ∼0.1% in the cerebellum. Therefore, based on aneuploidy, these adult mice with reduced life span and accelerated progeroid features are indistinguishable from age-matched, normal controls. Yet, during embryonic development, we found that Bub1b(H/H), but not Ercc1(-/Δ7) mice, have a significantly higher frequency of aneuploid nuclei relative to wild-type controls in the cerebral cortex, reaching a frequency as high as 40.3% for each chromosome tested. Aneuploid cells in these mutant mice are likely eliminated early in development through apoptosis and/or immune-mediated clearance mechanisms, which would explain the low levels of aneuploidy during adulthood in the cerebral cortex of Bub1b(H/H) mice. These results shed light on the mechanisms of removal of aneuploidy cells in vivo.
© The Author 2015. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Vijg J., Suh Y. (2013) Genome instability and aging. Ann. Rev. Physiol., 75, 645–668. - PubMed
-
- Hanks S., Coleman K., Reid S., Plaja A., Firth H., Fitzpatrick D., Kidd A., Mehes K., Nash R., Robin N. et al. (2004) Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat. Genetics, 36, 1159–1161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

