Resveratrol Protects PC12 Cell against 6-OHDA Damage via CXCR4 Signaling Pathway
- PMID: 26681969
- PMCID: PMC4670657
- DOI: 10.1155/2015/730121
Resveratrol Protects PC12 Cell against 6-OHDA Damage via CXCR4 Signaling Pathway
Abstract
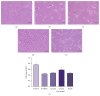
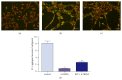
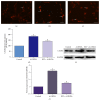
Resveratrol, herbal nonflavonoid polyphenolic compound naturally derived from grapes, has long been acknowledged to possess extensive biological and pharmacological properties including antioxidant and anti-inflammatory ones and may exert a neuroprotective effect on neuronal damage in neurodegenerative diseases. However, the underlying molecular mechanisms remain undefined. In the present study, we intended to investigate the neuroprotective effects of resveratrol against 6-OHDA-induced neurotoxicity of PC12 cells and further explore the possible mechanisms involved. For this purpose, PC12 cells were exposed to 6-OHDA in the presence of resveratrol (0, 12.5, 25, and 50 μM). The results showed that resveratrol increased cell viability, alleviated the MMP reduction, and reduced the number of apoptotic cells as measured by MTT assay, JC-1 staining, and Hoechst/PI double staining (all p < 0.01). Immunofluorescent staining and Western blotting revealed that resveratrol averts 6-OHDA induced CXCR4 upregulation (p < 0.01). Our results demonstrated that resveratrol could effectively protect PC12 cells from 6-OHDA-induced oxidative stress and apoptosis via CXCR4 signaling pathway.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

