The Tumorigenic Roles of the Cellular REDOX Regulatory Systems
- PMID: 26682014
- PMCID: PMC4670861
- DOI: 10.1155/2016/8413032
The Tumorigenic Roles of the Cellular REDOX Regulatory Systems
Abstract
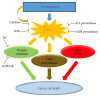
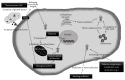
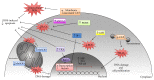
The cellular REDOX regulatory systems play a central role in maintaining REDOX homeostasis that is crucial for cell integrity, survival, and proliferation. To date, a substantial amount of data has demonstrated that cancer cells typically undergo increasing oxidative stress as the tumor develops, upregulating these important antioxidant systems in order to survive, proliferate, and metastasize under these extreme oxidative stress conditions. Since a large number of chemotherapeutic agents currently used in the clinic rely on the induction of ROS overload or change of ROS quality to kill the tumor, the cancer cell REDOX adaptation represents a significant obstacle to conventional chemotherapy. In this review we will first examine the different factors that contribute to the enhanced oxidative stress generally observed within the tumor microenvironment. We will then make a comprehensive assessment of the current literature regarding the main antioxidant proteins and systems that have been shown to be positively associated with tumor progression and chemoresistance. Finally we will make an analysis of commonly used chemotherapeutic drugs that induce ROS. The current knowledge of cancer cell REDOX adaptation raises the issue of developing novel and more effective therapies for these tumors that are usually resistant to conventional ROS inducing chemotherapy.
Figures
Similar articles
-
Redox Homeostasis and Cellular Antioxidant Systems: Crucial Players in Cancer Growth and Therapy.Oxid Med Cell Longev. 2016;2016:6235641. doi: 10.1155/2016/6235641. Epub 2016 Jun 21. Oxid Med Cell Longev. 2016. PMID: 27418953 Free PMC article. Review.
-
The causes of cancer revisited: "mitochondrial malignancy" and ROS-induced oncogenic transformation - why mitochondria are targets for cancer therapy.Mol Aspects Med. 2010 Apr;31(2):145-70. doi: 10.1016/j.mam.2010.02.008. Epub 2010 Mar 2. Mol Aspects Med. 2010. PMID: 20206201 Review.
-
Redox control in cancer development and progression.Mol Aspects Med. 2018 Oct;63:88-98. doi: 10.1016/j.mam.2018.02.003. Epub 2018 Mar 5. Mol Aspects Med. 2018. PMID: 29501614 Review.
-
Reactive oxygen species in redox cancer therapy.Cancer Lett. 2015 Oct 10;367(1):18-25. doi: 10.1016/j.canlet.2015.07.008. Epub 2015 Jul 14. Cancer Lett. 2015. PMID: 26187782 Review.
-
Reactive oxygen species: current knowledge and applications in cancer research and therapeutic.J Cell Biochem. 2008 May 15;104(2):657-67. doi: 10.1002/jcb.21655. J Cell Biochem. 2008. PMID: 18172854 Review.
Cited by
-
A Functional Food Inhibits Azoxymethane/Dextran Sulfate Sodium-Induced Inflammatory Colorectal Cancer in Mice.Onco Targets Ther. 2021 Feb 26;14:1465-1477. doi: 10.2147/OTT.S283465. eCollection 2021. Onco Targets Ther. 2021. PMID: 33664579 Free PMC article.
-
Protein kinase CK2 regulates redox homeostasis through NF-κB and Bcl-xL in cardiomyoblasts.Mol Cell Biochem. 2017 Dec;436(1-2):137-150. doi: 10.1007/s11010-017-3085-y. Epub 2017 Jun 8. Mol Cell Biochem. 2017. PMID: 28597245
-
N-Acetylcysteine Serves as Substrate of 3-Mercaptopyruvate Sulfurtransferase and Stimulates Sulfide Metabolism in Colon Cancer Cells.Cells. 2019 Aug 4;8(8):828. doi: 10.3390/cells8080828. Cells. 2019. PMID: 31382676 Free PMC article.
-
Chemotherapy Resistance: Role of Mitochondrial and Autophagic Components.Cancers (Basel). 2022 Mar 12;14(6):1462. doi: 10.3390/cancers14061462. Cancers (Basel). 2022. PMID: 35326612 Free PMC article. Review.
-
Epigenetic Therapeutics Targeting NRF2/KEAP1 Signaling in Cancer Oxidative Stress.Front Pharmacol. 2022 Jun 9;13:924817. doi: 10.3389/fphar.2022.924817. eCollection 2022. Front Pharmacol. 2022. PMID: 35754474 Free PMC article. Review.
References
-
- Marchissio M. J., Francés D. E. A., Carnovale C. E., Marinelli R. A. Mitochondrial aquaporin-8 knockdown in human hepatoma HepG2 cells causes ROS-induced mitochondrial depolarization and loss of viability. Toxicology and Applied Pharmacology. 2012;264(2):246–254. doi: 10.1016/j.taap.2012.08.005. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

