Coenzyme Q10 Attenuates High Glucose-Induced Endothelial Progenitor Cell Dysfunction through AMP-Activated Protein Kinase Pathways
- PMID: 26682233
- PMCID: PMC4670652
- DOI: 10.1155/2016/6384759
Coenzyme Q10 Attenuates High Glucose-Induced Endothelial Progenitor Cell Dysfunction through AMP-Activated Protein Kinase Pathways
Abstract
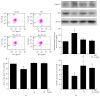
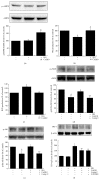
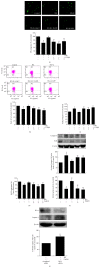
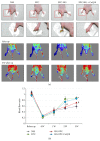
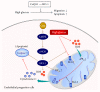
Coenzyme Q10 (CoQ10), an antiapoptosis enzyme, is stored in the mitochondria of cells. We investigated whether CoQ10 can attenuate high glucose-induced endothelial progenitor cell (EPC) apoptosis and clarified its mechanism. EPCs were incubated with normal glucose (5 mM) or high glucose (25 mM) environment for 3 days, followed by treatment with CoQ10 (10 μM) for 24 hr. Cell proliferation, nitric oxide (NO) production, and JC-1 assay were examined. The specific signal pathways of AMP-activated protein kinase (AMPK), eNOS/Akt, and heme oxygenase-1 (HO-1) were also assessed. High glucose reduced EPC functional activities, including proliferation and migration. Additionally, Akt/eNOS activity and NO production were downregulated in high glucose-stimulated EPCs. Administration of CoQ10 ameliorated high glucose-induced EPC apoptosis, including downregulation of caspase 3, upregulation of Bcl-2, and increase in mitochondrial membrane potential. Furthermore, treatment with CoQ10 reduced reactive oxygen species, enhanced eNOS/Akt activity, and increased HO-1 expression in high glucose-treated EPCs. These effects were negated by administration of AMPK inhibitor. Transplantation of CoQ10-treated EPCs under high glucose conditions into ischemic hindlimbs improved blood flow recovery. CoQ10 reduced high glucose-induced EPC apoptosis and dysfunction through upregulation of eNOS, HO-1 through the AMPK pathway. Our findings provide a potential treatment strategy targeting dysfunctional EPC in diabetic patients.
Figures


References
-
- Federman D. G., Bravata D. M., Kirsner R. S. Peripheral arterial disease: a systemic disease extending beyond the affected extremity. Geriatrics. 2004;59(4):29–32. - PubMed
-
- Vasa M., Fichtlscherer S., Aicher A., et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circulation Research. 2001;89(1):e1–e7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

