Multiparameter evaluation of in vivo gene delivery using ultrasound-guided, microbubble-enhanced sonoporation
- PMID: 26682505
- PMCID: PMC4724495
- DOI: 10.1016/j.jconrel.2015.12.001
Multiparameter evaluation of in vivo gene delivery using ultrasound-guided, microbubble-enhanced sonoporation
Abstract
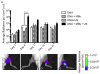
More than 1800 gene therapy clinical trials worldwide have targeted a wide range of conditions including cancer, cardiovascular diseases, and monogenic diseases. Biological (i.e. viral), chemical, and physical approaches have been developed to deliver nucleic acids into cells. Although viral vectors offer the greatest efficiency, they also raise major safety concerns including carcinogenesis and immunogenicity. The goal of microbubble-mediated sonoporation is to enhance the uptake of drugs and nucleic acids. Insonation of microbubbles is thought to facilitate two mechanisms for enhanced uptake: first, deflection of the cell membrane inducing endocytotic uptake, and second, microbubble jetting inducing the formation of pores in the cell membrane. We hypothesized that ultrasound could be used to guide local microbubble-enhanced sonoporation of plasmid DNA. With the aim of optimizing delivery efficiency, we used nonlinear ultrasound and bioluminescence imaging to optimize the acoustic pressure, microbubble concentration, treatment duration, DNA dosage, and number of treatments required for in vivo Luciferase gene expression in a mouse thigh muscle model. We found that mice injected with 50μg luciferase plasmid DNA and 5×10(5) microbubbles followed by ultrasound treatment at 1.4MHz, 200kPa, 100-cycle pulse length, and 540 Hz pulse repetition frequency (PRF) for 2min exhibited superior transgene expression compared to all other treatment groups. The bioluminescent signal measured for these mice on Day 4 post-treatment was 100-fold higher (p<0.0001, n=5 or 6) than the signals for controls treated with DNA injection alone, DNA and microbubble injection, or DNA injection and ultrasound treatment. Our results indicate that these conditions result in efficient gene delivery and prolonged gene expression (up to 21days) with no evidence of tissue damage or off-target delivery. We believe that these promising results bear great promise for the development of microbubble-enhanced sonoporation-induced gene therapies.
Keywords: “Gene therapy”; “Microbubbles”; “Sonoporation”; “Ultrasound”.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures

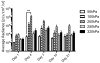
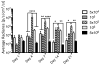
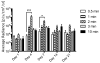
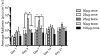

References
-
- Ginn SL, Alexander IE, Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2012 - an update. The journal of gene medicine. 2013;15:65–77. - PubMed
-
- Baum C, Kustikova O, Modlich U, Li ZX, Fehse B. Mutagenesis and oncogenesis by chromosomal insertion of gene transfer vectors. Hum Gene Ther. 2006;17:253–263. - PubMed
-
- Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11:S10–S17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

