Kainate receptor pore-forming and auxiliary subunits regulate channel block by a novel mechanism
- PMID: 26682513
- PMCID: PMC4818602
- DOI: 10.1113/JP271690
Kainate receptor pore-forming and auxiliary subunits regulate channel block by a novel mechanism
Abstract
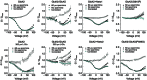
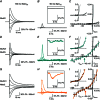
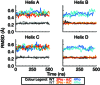
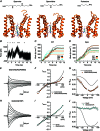
Key points: Kainate receptor heteromerization and auxiliary subunits, Neto1 and Neto2, attenuate polyamine ion-channel block by facilitating blocker permeation. Relief of polyamine block in GluK2/GluK5 heteromers results from a key proline residue that produces architectural changes in the channel pore α-helical region. Auxiliary subunits exert an additive effect to heteromerization, and thus relief of polyamine block is due to a different mechanism. Our findings have broad implications for work on polyamine block of other cation-selective ion channels.
Abstract: Channel block and permeation by cytoplasmic polyamines is a common feature of many cation-selective ion channels. Although the channel block mechanism has been studied extensively, polyamine permeation has been considered less significant as it occurs at extreme positive membrane potentials. Here, we show that kainate receptor (KAR) heteromerization and association with auxiliary proteins, Neto1 and Neto2, attenuate polyamine block by enhancing blocker permeation. Consequently, polyamine permeation and unblock occur at more negative and physiologically relevant membrane potentials. In GluK2/GluK5 heteromers, enhanced permeation is due to a single proline residue in GluK5 that alters the dynamics of the α-helical region of the selectivity filter. The effect of auxiliary proteins is additive, and therefore the structural basis of polyamine permeation and unblock is through a different mechanism. As native receptors are thought to assemble as heteromers in complex with auxiliary proteins, our data identify an unappreciated impact of polyamine permeation in shaping the signalling properties of neuronal KARs and point to a structural mechanism that may be shared amongst other cation-selective ion channels.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures




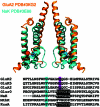
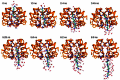

References
-
- Aizenman CD, Munoz‐Elias G & Cline HT (2002). Visually driven modulation of glutamatergic synaptic transmission is mediated by the regulation of intracellular polyamines. Neuron 34, 623–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

