The effects of acute and preventive migraine therapies in a mouse model of chronic migraine
- PMID: 26682574
- PMCID: PMC5557701
- DOI: 10.1177/0333102415623070
The effects of acute and preventive migraine therapies in a mouse model of chronic migraine
Abstract
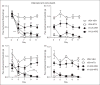
Background The development of novel migraine therapies has been slow, in part because of the small number of clinically relevant animal models. We have recently developed a new mouse model of chronic migraine using chronic intermittent nitroglycerin, a known human migraine trigger. The objective of this study was to validate this model by testing known and potential migraine-preventive treatments. Methods Migraine therapies were administered to male and female mice for 11 days. On day 3, mice were tested with nitroglycerin every second day for nine days. Basal and nitroglycerin-evoked mechanical hypersensitivity was evaluated using von Frey filaments. Results Chronic intermittent nitroglycerin produced acute hyperalgesia with each administration, and progressive and sustained basal hypersensitivity. The established preventive migraine therapy propranolol effectively blocked the development of acute and chronic nitroglycerin-induced hyperalgesia, while valproate had no effect. Potential migraine-preventive therapies were also tested: Amiloride inhibited nitroglycerin-induced acute and chronic hyperalgesia; while memantine was ineffective. We also tested the acute migraine therapy sumatriptan, which did not alter nitroglycerin-induced hyperalgesia, but instead resulted in acute and chronic hyperalgesia similar to that observed following nitroglycerin administration. Conclusions This study establishes the chronic nitroglycerin model as an additional screening tool to test novel migraine-preventive therapies.
Keywords: Allodynia; pain; prophylactic; triptan.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

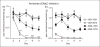


References
-
- Bigal ME, Serrano D, Reed M, et al. Chronic migraine in the population: Burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71:559–566. - PubMed
-
- Schwedt TJ. Chronic migraine. BMJ. 2014;348:g1416. - PubMed
-
- The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33:629–808. - PubMed
-
- Stovner L, Hagen K, Jensen R, et al. The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. - PubMed
-
- Victor TW, Hu X, Campbell JC, et al. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia. 2010;30:1065–1072. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

