Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures
- PMID: 26682988
- PMCID: PMC4859820
- DOI: 10.1016/j.immuni.2015.11.012
Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures
Abstract
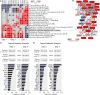
Systems approaches have been used to describe molecular signatures driving immunity to influenza vaccination in humans. Whether such signatures are similar across multiple seasons and in diverse populations is unknown. We applied systems approaches to study immune responses in young, elderly, and diabetic subjects vaccinated with the seasonal influenza vaccine across five consecutive seasons. Signatures of innate immunity and plasmablasts correlated with and predicted influenza antibody titers at 1 month after vaccination with >80% accuracy across multiple seasons but were not associated with the longevity of the response. Baseline signatures of lymphocyte and monocyte inflammation were positively and negatively correlated, respectively, with antibody responses at 1 month. Finally, integrative analysis of microRNAs and transcriptomic profiling revealed potential regulators of vaccine immunity. These results identify shared vaccine-induced signatures across multiple seasons and in diverse populations and might help guide the development of next-generation vaccines that provide persistent immunity against influenza.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




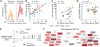

References
-
- Almeida-Oliveira A, Smith-Carvalho M, Porto LC, Cardoso-Oliveira J, Ribeiro AdosS, Falcão RR, Abdelhay E, Bouzas LF, Thuler LCS, Ornellas MH, Diamond HR. Age-related changes in natural killer cell receptors from childhood through old age. Hum Immunol. 2011;72:319–329. - PubMed
-
- Béziat V, Duffy D, Quoc SN, Le Garff-Tavernier M, Decocq J, Combadière B, Debré P, Vieillard V. CD56brightCD16+ NK cells: a functional intermediate stage of NK cell differentiation. J Immunol. 2011;186:6753–6761. - PubMed
-
- Bhattacharya S, Andorf S, Gomes L, Dunn P, Schaefer H, Pontius J, Berger P, Desborough V, Smith T, Campbell J, et al. ImmPort: disseminating data to the public for the future of immunology. Immunol Res. 2014;58:234–239. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- R37 AI048638/AI/NIAID NIH HHS/United States
- U19 AI090023/AI/NIAID NIH HHS/United States
- R37 AG023717/AG/NIA NIH HHS/United States
- R37DK057665/DK/NIDDK NIH HHS/United States
- U19AI090023/AI/NIAID NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- U19AI057266/AI/NIAID NIH HHS/United States
- HHSN272201400004C/AI/NIAID NIH HHS/United States
- U54AI057157/AI/NIAID NIH HHS/United States
- R37 DK057665/DK/NIDDK NIH HHS/United States
- HHSN272201400004C/AI/NIAID NIH HHS/United States
- R37AI48638/AI/NIAID NIH HHS/United States
- U54 AI057157/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- R38 AI140299/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

