Modeling Lymph Flow and Fluid Exchange with Blood Vessels in Lymph Nodes
- PMID: 26683026
- PMCID: PMC4685511
- DOI: 10.1089/lrb.2015.0028
Modeling Lymph Flow and Fluid Exchange with Blood Vessels in Lymph Nodes
Abstract
Background: Lymph nodes (LNs) are positioned strategically throughout the body as critical mediators of lymph filtration and immune response. Lymph carries cytokines, antigens, and cells to the downstream LNs, and their effective delivery to the correct location within the LN directly impacts the quality and quantity of immune response. Despite the importance of this system, the flow patterns in LN have never been quantified, in part because experimental characterization is so difficult.
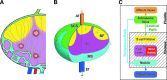
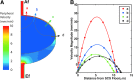
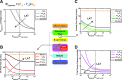
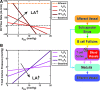
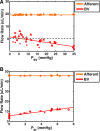
Methods and results: To achieve a more quantitative knowledge of LN flow, a computational flow model has been developed based on the mouse popliteal LN, allowing for a parameter sensitivity analysis to identify the important system characteristics. This model suggests that about 90% of the lymph takes a peripheral path via the subcapsular and medullary sinuses, while fluid perfusing deeper into the paracortex is sequestered by parenchymal blood vessels. Fluid absorption by these blood vessels under baseline conditions was driven mainly by oncotic pressure differences between lymph and blood, although the magnitude of fluid transfer is highly dependent on blood vessel surface area. We also predict that the hydraulic conductivity of the medulla, a parameter that has never been experimentally measured, should be at least three orders of magnitude larger than that of the paracortex to ensure physiologic pressures across the node.
Conclusions: These results suggest that structural changes in the LN microenvironment, as well as changes in inflow/outflow conditions, dramatically alter the distribution of lymph, cytokines, antigens, and cells within the LN, with great potential for modulating immune response.
Figures





References
-
- Gashev AA, Davis MJ, Delp MD, Zawieja DC. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 2004;11:477–492 - PubMed
-
- Koller A, Mizuno R, Kaley G. Flow reduces the amplitude and increases the frequency of lymphatic vasomotion: Role of endothelial prostanoids. Am J Physiol 1999; 27:R1683–1689 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

