Expression of Lactate Dehydrogenase in Aspergillus niger for L-Lactic Acid Production
- PMID: 26683313
- PMCID: PMC4684279
- DOI: 10.1371/journal.pone.0145459
Expression of Lactate Dehydrogenase in Aspergillus niger for L-Lactic Acid Production
Abstract
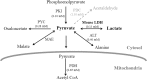
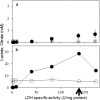
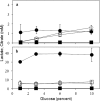
Different engineered organisms have been used to produce L-lactate. Poor yields of lactate at low pH and expensive downstream processing remain as bottlenecks. Aspergillus niger is a prolific citrate producer and a remarkably acid tolerant fungus. Neither a functional lactate dehydrogenase (LDH) from nor lactate production by A. niger is reported. Its genome was also investigated for the presence of a functional ldh. The endogenous A. niger citrate synthase promoter relevant to A. niger acidogenic metabolism was employed to drive constitutive expression of mouse lactate dehydrogenase (mldhA). An appraisal of different branches of the A. niger pyruvate node guided the choice of mldhA for heterologous expression. A high copy number transformant C12 strain, displaying highest LDH specific activity, was analyzed under different growth conditions. The C12 strain produced 7.7 g/l of extracellular L-lactate from 60 g/l of glucose, in non-neutralizing minimal media. Significantly, lactate and citrate accumulated under two different growth conditions. Already an established acidogenic platform, A. niger now promises to be a valuable host for lactate production.
Conflict of interest statement
Figures


References
-
- Zhan ZY, Jin B, Kelly JM. Production of lactic acid from renewable materials by Rhizopus fungi. Biochem Eng J. 2007; 35: 251–63.
-
- Ishida N, Saitoh S, Onishi T, Tokuhiro K, Nagamori E, Kitamoto K, et al. The effect of pyruvate decarboxylase gene knockout in Saccharomyces cerevisiae on L-lactic acid production. Biosci Biotechnol Biochem. 2006; 70: 1148–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

