Computational Models Describing Possible Mechanisms for Generation of Excessive Beta Oscillations in Parkinson's Disease
- PMID: 26683341
- PMCID: PMC4684204
- DOI: 10.1371/journal.pcbi.1004609
Computational Models Describing Possible Mechanisms for Generation of Excessive Beta Oscillations in Parkinson's Disease
Abstract
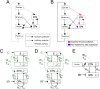
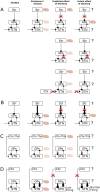
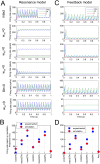
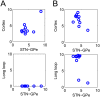
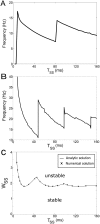
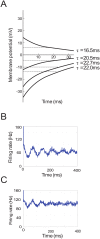
In Parkinson's disease, an increase in beta oscillations within the basal ganglia nuclei has been shown to be associated with difficulty in movement initiation. An important role in the generation of these oscillations is thought to be played by the motor cortex and by a network composed of the subthalamic nucleus (STN) and the external segment of globus pallidus (GPe). Several alternative models have been proposed to describe the mechanisms for generation of the Parkinsonian beta oscillations. However, a recent experimental study of Tachibana and colleagues yielded results which are challenging for all published computational models of beta generation. That study investigated how the presence of beta oscillations in a primate model of Parkinson's disease is affected by blocking different connections of the STN-GPe circuit. Due to a large number of experimental conditions, the study provides strong constraints that any mechanistic model of beta generation should satisfy. In this paper we present two models consistent with the data of Tachibana et al. The first model assumes that Parkinsonian beta oscillation are generated in the cortex and the STN-GPe circuits resonates at this frequency. The second model additionally assumes that the feedback from STN-GPe circuit to cortex is important for maintaining the oscillations in the network. Predictions are made about experimental evidence that is required to differentiate between the two models, both of which are able to reproduce firing rates, oscillation frequency and effects of lesions carried out by Tachibana and colleagues. Furthermore, an analysis of the models reveals how the amplitude and frequency of the generated oscillations depend on parameters.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Brown P, Oliviero A, Mazzone P, Insola A, Tonali P, Di Lazzaro V. Dopamine Dependency of Oscillations between Subthalamic Nucleus and Pallidum in Parkinson’s Disease. J Neurosci. 2001. February;21(3):1033–1038. Available from: http://www.jneurosci.org/content/21/3/1033.abstract. - PMC - PubMed
-
- Kühn AA, Kupsch A, Schneider GH, Brown P. Reduction in subthalamic 8–35 Hz oscillatory activity correlates with clinical improvement in Parkinson’s disease. Eur J Neurosci. 2006. April;23(7):1956–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16623853. 10.1111/j.1460-9568.2006.04717.x - DOI - PubMed
-
- Nambu A, Tachibana Y. Mechanism of parkinsonian neuronal oscillations in the primate basal ganglia: some considerations based on our recent work. Front Syst Neurosci. 2014. January;8:74 Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4033056&tool=p.... 10.3389/fnsys.2014.00074 - DOI - PMC - PubMed
-
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002. October;25(10):525–31. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12220881. 10.1016/S0166-2236(02)02235-X - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

