Bile Acid Signaling Is Involved in the Neurological Decline in a Murine Model of Acute Liver Failure
- PMID: 26683664
- PMCID: PMC4729266
- DOI: 10.1016/j.ajpath.2015.10.005
Bile Acid Signaling Is Involved in the Neurological Decline in a Murine Model of Acute Liver Failure
Abstract
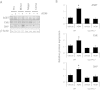
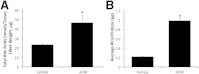
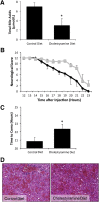
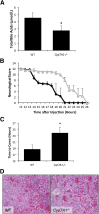
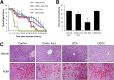
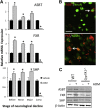
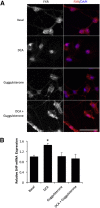
Hepatic encephalopathy is a serious neurological complication of liver failure. Serum bile acids are elevated after liver damage and may disrupt the blood-brain barrier and enter the brain. Our aim was to assess the role of serum bile acids in the neurological complications after acute liver failure. C57Bl/6 or cytochrome p450 7A1 knockout (Cyp7A1(-/-)) mice were fed a control, cholestyramine-containing, or bile acid-containing diet before azoxymethane (AOM)-induced acute liver failure. In parallel, mice were given an intracerebroventricular infusion of farnesoid X receptor (FXR) Vivo-morpholino before AOM injection. Liver damage, neurological decline, and molecular analyses of bile acid signaling were performed. Total bile acid levels were increased in the cortex of AOM-treated mice. Reducing serum bile acids via cholestyramine feeding or using Cyp7A1(-/-) mice reduced bile acid levels and delayed AOM-induced neurological decline, whereas cholic acid or deoxycholic acid feeding worsened AOM-induced neurological decline. The expression of bile acid signaling machinery apical sodium-dependent bile acid transporter, FXR, and small heterodimer partner increased in the frontal cortex, and blocking FXR signaling delayed AOM-induced neurological decline. In conclusion, circulating bile acids may play a pathological role during hepatic encephalopathy, although precisely how they dysregulate normal brain function is unknown. Strategies to minimize serum bile acid concentrations may reduce the severity of neurological complications associated with liver failure.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Butterworth R.F. Hepatic encephalopathy: a central neuroinflammatory disorder? Hepatology. 2011;53:1372–1376. - PubMed
-
- Hazell A.S., Butterworth R.F. Hepatic encephalopathy: an update of pathophysiologic mechanisms. Proc Soc Exp Biol Med. 1999;222:99–112. - PubMed
-
- Kramer L., Kodras K. Detoxification as a treatment goal in hepatic failure. Liver Int. 2011;31 Suppl 3:1–4. - PubMed
-
- Ostapowicz G., Fontana R.J., Schiodt F.V., Larson A., Davern T.J., Han S.H., McCashland T.M., Shakil A.O., Hay J.E., Hynan L., Crippin J.S., Blei A.T., Samuel G., Reisch J., Lee W.M. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–954. - PubMed
-
- St-Pierre M.V., Kullak-Ublick G.A., Hagenbuch B., Meier P.J. Transport of bile acids in hepatic and non-hepatic tissues. J Exp Biol. 2001;204:1673–1686. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

