Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B
- PMID: 26684441
- PMCID: PMC4766024
- DOI: 10.1053/j.gastro.2015.11.050
Hepatitis B Virus--Specific and Global T-Cell Dysfunction in Chronic Hepatitis B
Abstract
Background & aims: T cells play a critical role in viral infection. We examined whether T-cell effector and regulatory responses can define clinical stages of chronic hepatitis B (CHB).
Methods: We enrolled 200 adults with CHB who participated in the National Institutes of Health-supported Hepatitis B Research Network from 2011 through 2013 and 20 uninfected individuals (controls). Peripheral blood lymphocytes from these subjects were analyzed for T-cell responses (proliferation and production of interferon gamma and interleukin 10) to overlapping hepatitis B virus (HBV) peptides (preS, S, preC, core, and reverse transcriptase), influenza matrix peptides, and lipopolysaccharide. T-cell expression of regulatory markers FOXP3, programmed death-1, and cytotoxic T lymphocyte-associated antigen-4 was examined by flow cytometry. Immune measures were compared with clinical parameters, including physician-defined immune-active, immune-tolerant, or inactive CHB phenotypes, in a blinded fashion.
Results: Compared with controls, patients with CHB had weak T-cell proliferative, interferon gamma, and interleukin 10 responses to HBV, with increased frequency of circulating FOXP3(+)CD127(-) regulatory T cells and CD4(+) T-cell expression of programmed death-1 and cytotoxic T lymphocyte-associated antigen-4. T-cell measures did not clearly distinguish between clinical CHB phenotypes, although the HBV core-specific T-cell response was weaker in hepatitis B e antigen (HBeAg)(+) than HBeAg(-) patients (percent responders: 3% vs 23%; P = .00008). Although in vitro blockade of programmed death-1 or cytotoxic T lymphocyte-associated antigen-4 increased T-cell responses to HBV, the effect was weaker in HBeAg(+) than HBeAg(-) patients. Furthermore, T-cell responses to influenza and lipopolysaccharide were weaker in CHB patients than controls.
Conclusions: HBV persists with virus-specific and global T-cell dysfunction mediated by multiple regulatory mechanisms, including circulating HBeAg, but without distinct T-cell-based immune signatures for clinical phenotypes. These findings suggest additional T-cell-independent or regulatory mechanisms of CHB pathogenesis that warrant further investigation.
Keywords: HBRN; IFN; IL10; LPS.
Copyright © 2016. Published by Elsevier Inc.
Figures

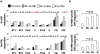

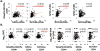

References
-
- Hoofnagle JH, Doo E, Liang TJ, et al. Management of hepatitis B: summary of a clinical research workshop. Hepatology. 2007;45:1056–75. - PubMed
-
- Chisari FV, Ferrari C. Hepatitis B virus immunopathogenesis. Annu Rev Immunol. 1995;13:29–60. - PubMed
-
- Liang R. How I treat and monitor viral hepatitis B infection in patients receiving intensive immunosuppressive therapies or undergoing hematopoietic stem cell transplantation. Blood. 2009;113:3147–53. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UL1 TR001111/TR/NCATS NIH HHS/United States
- P30 DK026743/DK/NIDDK NIH HHS/United States
- UL1 TR000058/TR/NCATS NIH HHS/United States
- DK082867/DK/NIDDK NIH HHS/United States
- DK082863/DK/NIDDK NIH HHS/United States
- I01 BX000649/BX/BLRD VA/United States
- M01-RR00040/RR/NCRR NIH HHS/United States
- UO-1DK082866/DK/NIDDK NIH HHS/United States
- DK082943/DK/NIDDK NIH HHS/United States
- UL1 TR000433/TR/NCATS NIH HHS/United States
- R01-AI-47519/AI/NIAID NIH HHS/United States
- DK082871/DK/NIDDK NIH HHS/United States
- U01 DK082919/DK/NIDDK NIH HHS/United States
- U01 DK082927/DK/NIDDK NIH HHS/United States
- U01 DK082872/DK/NIDDK NIH HHS/United States
- U01 DK082943/DK/NIDDK NIH HHS/United States
- UL1TR001111/TR/NCATS NIH HHS/United States
- DK082866/DK/NIDDK NIH HHS/United States
- DK082919/DK/NIDDK NIH HHS/United States
- VA999999/ImVA/Intramural VA/United States
- UL1RR024986)/RR/NCRR NIH HHS/United States
- P30 DK050306/DK/NIDDK NIH HHS/United States
- M01 RR000040/RR/NCRR NIH HHS/United States
- DK082864/DK/NIDDK NIH HHS/United States
- R01 AI047519/AI/NIAID NIH HHS/United States
- U01 DK082923/DK/NIDDK NIH HHS/United States
- U01 DK082871/DK/NIDDK NIH HHS/United States
- U01 DK082944/DK/NIDDK NIH HHS/United States
- U01 DK082864/DK/NIDDK NIH HHS/United States
- DK082927/DK/NIDDK NIH HHS/United States
- UL1TR000058/TR/NCATS NIH HHS/United States
- UL1 TR002489/TR/NCATS NIH HHS/United States
- P30DK50306/DK/NIDDK NIH HHS/United States
- U01 DK082867/DK/NIDDK NIH HHS/United States
- U01 DK082843/DK/NIDDK NIH HHS/United States
- U01 DK082863/DK/NIDDK NIH HHS/United States
- DK082874/DK/NIDDK NIH HHS/United States
- DK082923/DK/NIDDK NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- UL1TR000004/TR/NCATS NIH HHS/United States
- DK 082843/DK/NIDDK NIH HHS/United States
- U01 DK082874/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- U01 DK082866/DK/NIDDK NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- U01DK082944/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

