Developmentally arrested Austrofundulus limnaeus embryos have changes in post-translational modifications of histone H3
- PMID: 26685169
- PMCID: PMC6514462
- DOI: 10.1242/jeb.131862
Developmentally arrested Austrofundulus limnaeus embryos have changes in post-translational modifications of histone H3
Abstract
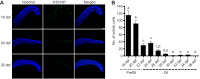
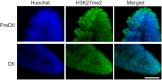
Although vertebrate embryogenesis is typically a continuous and dynamic process, some embryos have evolved mechanisms to developmentally arrest. The embryos of Austrofundulus limnaeus, a killifish that resides in ephemeral ponds, routinely enter diapause II (DII), a reversible developmental arrest promoted by endogenous cues rather than environmental stress. DII, which starts at 24-26 days post-fertilization and can persist for months, is characterized by a significant decline in heart rate and an arrest of development and differentiation. Thus, A. limnaeus is a unique model to study epigenetic features associated with embryonic arrest. To investigate chromosome structures associated with mitosis or gene expression, we examined the post-translational modifications of histone H3 (phosphorylation of serine 10, mono-, di- and tri-methylation of lysine 4 or 27) in preDII, DII and postDII embryos. As seen by microscopy analysis, DII embryos have a significant decrease in the H3S10P marker for mitotic nuclei and an inner nuclear membrane localization of the H3K27me2 marker associated with silencing of gene expression. ELISA experiments reveal that the levels of methylation at H3K4 and H3K27 are significantly different between preDII, DII and postDII embryos, indicating that there are molecular differences between embryos of different chronological age and stage of development. Furthermore, in DII embryos relative to preDII embryos, there are differences in the level of H3K27me3 and H3K4me3, which may reflect critical chromatin remodeling that occurs prior to arrest of embryogenesis. This work helps lay a foundation for chromatin analysis of vertebrate embryo diapause, an intriguing yet greatly understudied phenomenon.
Keywords: Chromatin; Diapause; Killifish.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures



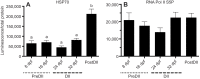
Similar articles
-
The genome of Austrofundulus limnaeus offers insights into extreme vertebrate stress tolerance and embryonic development.BMC Genomics. 2018 Feb 20;19(1):155. doi: 10.1186/s12864-018-4539-7. BMC Genomics. 2018. PMID: 29463212 Free PMC article.
-
Cell cycle arrest associated with anoxia-induced quiescence, anoxic preconditioning, and embryonic diapause in embryos of the annual killifish Austrofundulus limnaeus.J Comp Physiol B. 2012 Oct;182(7):909-20. doi: 10.1007/s00360-012-0672-9. Epub 2012 May 9. J Comp Physiol B. 2012. PMID: 22570106 Free PMC article.
-
Extreme tolerance and developmental buffering of UV-C induced DNA damage in embryos of the annual killifish Austrofundulus limnaeus.J Exp Zool A Ecol Genet Physiol. 2015 Jan;323(1):10-30. doi: 10.1002/jez.1890. Epub 2014 Nov 11. J Exp Zool A Ecol Genet Physiol. 2015. PMID: 25387429
-
Cell cycle regulation during development and dormancy in embryos of the annual killifish Austrofundulus limnaeus.Cell Cycle. 2012 May 1;11(9):1697-704. doi: 10.4161/cc.19881. Epub 2012 May 1. Cell Cycle. 2012. PMID: 22531486 Free PMC article. Review.
-
Dispersion/reaggregation in early development of annual killifishes: Phylogenetic distribution and evolutionary significance of a unique feature.Dev Biol. 2018 Oct 1;442(1):69-79. doi: 10.1016/j.ydbio.2018.07.015. Epub 2018 Jul 21. Dev Biol. 2018. PMID: 30040922 Review.
Cited by
-
Using diapause as a platform to understand the biology of dormancy.Open Biol. 2025 Aug;15(8):250104. doi: 10.1098/rsob.250104. Epub 2025 Aug 20. Open Biol. 2025. PMID: 40829645 Free PMC article. Review.
-
Epigenetics and seasonal timing in animals: a concise review.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024 Jul;210(4):565-574. doi: 10.1007/s00359-023-01673-3. Epub 2023 Sep 11. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2024. PMID: 37695537 Free PMC article. Review.
References
-
- Agger K., Cloos P. A. C., Rudkjaer L., Williams K., Andersen G., Christensen J. and Helin K. (2009). The H3K27me3 demethylase JMJD3 contributes to the activation of the INK4A-ARF locus in response to oncogene- and stress-induced senescence. Genes Dev. 23, 1171-1176. 10.1101/gad.510809 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

