Effects of Chrysotile Exposure in Human Bronchial Epithelial Cells: Insights into the Pathogenic Mechanisms of Asbestos-Related Diseases
- PMID: 26685284
- PMCID: PMC4892914
- DOI: 10.1289/ehp.1409627
Effects of Chrysotile Exposure in Human Bronchial Epithelial Cells: Insights into the Pathogenic Mechanisms of Asbestos-Related Diseases
Abstract
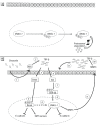
Background: Chrysotile asbestos accounts for > 90% of the asbestos used worldwide, and exposure is associated with asbestosis (asbestos-related fibrosis) and other malignancies; however, the molecular mechanisms involved are not fully understood. A common pathogenic mechanism for these malignancies is represented by epithelial-mesenchymal transition (EMT), through which epithelial cells undergo a morphological transformation to assume a mesenchymal phenotype. In the present work, we propose that chrysotile asbestos induces EMT through a mechanism involving a signaling pathway mediated by tranforming growth factor beta (TGF-β).
Objectives: We investigated the role of chrysotile asbestos in inducing EMT in order to elucidate the molecular mechanisms involved in this event.
Methods: Human bronchial epithelial cells (BEAS-2B) were incubated with 1 μg/cm2 chrysotile asbestos for ≤ 72 hr, and several markers of EMT were investigated. Experiments with specific inhibitors for TGF-β, glycogen synthase kinase-3β (GSK-3β), and Akt were performed to confirm their involvement in asbestos-induced EMT. Real-time polymerase chain reaction (PCR), Western blotting, and gelatin zymography were performed to detect mRNA and protein level changes for these markers.
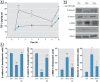
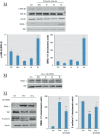
Results: Chrysotile asbestos activated a TGF-β-mediated signaling pathway, implicating the contributions of Akt, GSK-3β, and SNAIL-1. The activation of this pathway in BEAS-2B cells was associated with a decrease in epithelial markers (E-cadherin and β-catenin) and an increase in mesenchymal markers (α-smooth muscle actin, vimentin, metalloproteinases, and fibronectin).
Conclusions: Our findings suggest that chrysotile asbestos induces EMT, a common event in asbestos-related diseases, at least in part by eliciting the TGF-β-mediated Akt/GSK-3β/SNAIL-1 pathway.
Citation: Gulino GR, Polimeni M, Prato M, Gazzano E, Kopecka J, Colombatto S, Ghigo D, Aldieri E. 2016. Effects of chrysotile exposure in human bronchial epithelial cells: insights into the pathogenic mechanisms of asbestos-related diseases. Environ Health Perspect 124:776-784; http://dx.doi.org/10.1289/ehp.1409627.
Conflict of interest statement
This paper is dedicated to the memory of D.G., who passed away on 7 October 2015. We all remember our friend as a master in life and science, and we hope he will continue to help us from the place where he is now.
The authors declare they have no actual or potential competing financial interests.
Figures



References
-
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132(14):3151–3161. - PubMed
-
- Bass DA, Parce JW, Dechatelet LR, Szejda P, Seeds MC, Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983;130(4):1910–1917. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

