The combination of FLT3 and DNA methyltransferase inhibition is synergistically cytotoxic to FLT3/ITD acute myeloid leukemia cells
- PMID: 26686245
- PMCID: PMC5244408
- DOI: 10.1038/leu.2015.346
The combination of FLT3 and DNA methyltransferase inhibition is synergistically cytotoxic to FLT3/ITD acute myeloid leukemia cells
Abstract
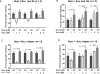
Effective treatment regimens for elderly acute myeloid leukemia (AML) patients harboring internal tandem duplication mutations in the FMS-like tyrosine kinase-3 (FLT3) gene (FLT3/ITD) are lacking and represent a significant unmet need. Recent data on the effects of FLT3 tyrosine kinase inhibitors on FLT3/ITD(+) AML showed promising clinical activity, including in elderly patients. DNA methyltransferase (DNMT) inhibitors such as decitabine (5-aza-2-deoxycytidine, DEC) and 5-azacitidine (AZA) demonstrated clinical benefit in AML, are well tolerated and are associated with minimal increases in FLT3 ligand, which can represent a potential resistance mechanism to FLT3 inhibitors. In addition, both FLT3 and DNMT inhibition are associated with the induction of terminal differentiation of myeloid blasts. Consequently, there is a strong theoretical rationale for combining FLT3 and DNMT inhibition for FLT3/ITD(+) AML. We therefore sought to study the anti-leukemic effects of DEC, AZA and FLT3 inhibitors, either as single agents or in combination, on AML cell lines and primary cells derived from newly diagnosed and relapsed AML patients. Our studies indicate that combined treatment using FLT3 inhibition and hypomethylation confers synergistic anti-leukemic effects, including apoptosis, growth inhibition and differentiation. The simultaneous administration of AZA and FLT3 inhibition appears to be the most efficacious combination in this regard. These drugs may provide a novel therapeutic approach for FLT3/ITD(+) AML, in particular for older patients.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

