Medicinal chemistry of adenosine, P2Y and P2X receptors
- PMID: 26686393
- PMCID: PMC4871727
- DOI: 10.1016/j.neuropharm.2015.12.001
Medicinal chemistry of adenosine, P2Y and P2X receptors
Abstract
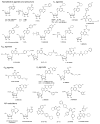
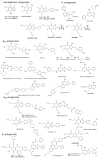
Pharmacological tool compounds are now available to define action at the adenosine (ARs), P2Y and P2X receptors. We present a selection of the most commonly used agents to study purines in the nervous system. Some of these compounds, including A1 and A3 AR agonists, P2Y1R and P2Y12R antagonists, and P2X3, P2X4 and P2X7 antagonists, are potentially of clinical use in treatment of disorders of the nervous system, such as chronic pain, neurodegeneration and brain injury. Agonists of the A2AAR and P2Y2R are already used clinically, P2Y12R antagonists are widely used antithrombotics and an antagonist of the A2AAR is approved in Japan for treating Parkinson's disease. The selectivity defined for some of the previously introduced compounds has been revised with updated pharmacological characterization, for example, various AR agonists and antagonists were deemed A1AR or A3AR selective based on human data, but species differences indicated a reduction in selectivity ratios in other species. Also, many of the P2R ligands still lack bioavailability due to charged groups or hydrolytic (either enzymatic or chemical) instability. X-ray crystallographic structures of AR and P2YRs have shifted the mode of ligand discovery to structure-based approaches rather than previous empirical approaches. The X-ray structures can be utilized either for in silico screening of chemically diverse libraries for the discovery of novel ligands or for enhancement of the properties of known ligands by chemical modification. Although X-ray structures of the zebrafish P2X4R have been reported, there is scant structural information about ligand recognition in these trimeric ion channels. In summary, there are definitive, selective agonists and antagonists for all of the ARs and some of the P2YRs; while the pharmacochemistry of P2XRs is still in nascent stages. The therapeutic potential of selectively modulating these receptors is continuing to gain interest in such fields as cancer, inflammation, pain, diabetes, ischemic protection and many other conditions. This article is part of the Special Issue entitled 'Purines in Neurodegeneration and Neuroregeneration'.
Keywords: 2-MeSADP, (PubChem CID: 121990); A-740003, (PubChem CID: 23232014); ATP; Agonists; Antagonists; DPCPX, (PubChem CID: 1329); GPCR; IB-MECA, (PubChem CID: 123683); Ion channel; LUF6000, (PubChem CID: 11711282); MRS2500, (PubChem CID: 44448831); Nucleosides; Nucleotides; PPTN, (PubChem CID: 42611190); PSB-1114, (PubChem CID: 52952605); PSB-603, (PubChem CID: 44185871); SCH442416, (PubChem CID: 10668061).
Published by Elsevier Ltd.
Figures
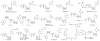

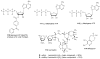
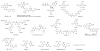

References
-
- Alexander K, Niforatos W, Bianchi B, Burgard EC, Lynch KJ, Kowaluk EA, Jarvis MF, van Biesen T. Allosteric modulation and accelerated resensitization of human P2X(3) receptors by cibacron blue. J Pharmacol Exp Ther. 1999;291:1135–1142. - PubMed
-
- Al-Rashida M, Iqbal J. Therapeutic potentials of ecto-nucleoside triphosphate diphosphohydrolase, ecto-nucleotide pyrophosphatase/phosphodiesterase, ecto-5′-nucleotidase, and alkaline phosphatase inhibitors. Med Res Rev. 2014;34:703–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

