Drug-protein hydrogen bonds govern the inhibition of the ATP hydrolysis of the multidrug transporter P-glycoprotein
- PMID: 26686578
- PMCID: PMC4753104
- DOI: 10.1016/j.bcp.2015.12.007
Drug-protein hydrogen bonds govern the inhibition of the ATP hydrolysis of the multidrug transporter P-glycoprotein
Abstract
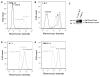
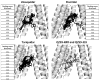
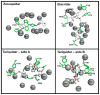
P-glycoprotein (P-gp) is a member of the ATP-binding cassette transporter superfamily. This multidrug transporter utilizes energy from ATP hydrolysis for the efflux of a variety of hydrophobic and amphipathic compounds including anticancer drugs. Most of the substrates and modulators of P-gp stimulate its basal ATPase activity, although some inhibit it. The molecular mechanisms that are in play in either case are unknown. In this report, mutagenesis and molecular modeling studies of P-gp led to the identification of a pair of phenylalanine-tyrosine structural motifs in the transmembrane region that mediate the inhibition of ATP hydrolysis by certain drugs (zosuquidar, elacridar and tariquidar), with high affinity (IC50's ranging from 10 to 30nM). Upon mutation of any of these residues, drugs that inhibit the ATPase activity of P-gp switch to stimulation of the activity. Molecular modeling revealed that the phenylalanine residues F978 and F728 interact with tyrosine residues Y953 and Y310, respectively, in an edge-to-face conformation, which orients the tyrosines in such a way that they establish hydrogen-bond contacts with the inhibitor. Biochemical investigations along with transport studies in intact cells showed that the inhibitors bind at a high affinity site to produce inhibition of ATP hydrolysis and transport function. Upon mutation, they bind at lower affinity sites, stimulating ATP hydrolysis and only poorly inhibiting transport. These results also reveal that screening chemical compounds for their ability to inhibit the basal ATP hydrolysis can be a reliable tool to identify modulators with high affinity for P-gp.
Keywords: ABC transporter; Calcein-AM (PubChem CID: 4126474); Cyclosporine A (PubChem CID: 5284373); Daunorubicin (PubChem CID: 30323); Drug-binding site; Elacridar (PubChem CID: 119373); Modulators; Multidrug resistance; Nilotinib (PubChem CID: 644241); Rhodamine 123 (PubChem CID: 65217); Structural motifs; Tariquidar (PubChem CID: 148201); Valinomycin (PubChem CID: 5649); Verapamil (PubChem CID: 62969); Zosuquidar (PubChem CID: 153997).
Published by Elsevier Inc.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

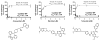






References
-
- Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annual Review of Pharmacology and Toxicology. 1999;39:361–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

