Condensin Relocalization from Centromeres to Chromosome Arms Promotes Top2 Recruitment during Anaphase
- PMID: 26686624
- PMCID: PMC4695335
- DOI: 10.1016/j.celrep.2015.11.041
Condensin Relocalization from Centromeres to Chromosome Arms Promotes Top2 Recruitment during Anaphase
Abstract
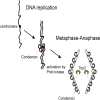
Condensin is a conserved chromosomal complex necessary to promote mitotic chromosome condensation and sister chromatid resolution during anaphase. Here, we report that yeast condensin binds to replicated centromere regions. We show that centromeric condensin relocalizes to chromosome arms as cells undergo anaphase segregation. We find that condensin relocalization is initiated immediately after the bipolar attachment of sister kinetochores to spindles and requires Polo kinase activity. Moreover, condensin localization during anaphase involves a higher binding rate on DNA and temporally overlaps with condensin's DNA overwinding activity. Finally, we demonstrate that topoisomerase 2 (Top2) is also recruited to chromosome arms during anaphase in a condensin-dependent manner. Our results uncover a functional relation between condensin and Top2 during anaphase to mediate chromosome segregation.
Copyright © 2015 The Authors. Published by Elsevier Inc. All rights reserved.
Figures



References
-
- Baxter J., Aragón L. A model for chromosome condensation based on the interplay between condensin and topoisomerase II. Trends Genet. 2012;28:110–117. - PubMed
-
- Baxter J., Sen N., Martínez V.L., De Carandini M.E., Schvartzman J.B., Diffley J.F., Aragón L. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science. 2011;331:1328–1332. - PubMed
-
- Bazett-Jones D.P., Kimura K., Hirano T. Efficient supercoiling of DNA by a single condensin complex as revealed by electron spectroscopic imaging. Mol. Cell. 2002;9:1183–1190. - PubMed
-
- Cuylen S., Metz J., Hruby A., Haering C.H. Entrapment of chromosomes by condensin rings prevents their breakage during cytokinesis. Dev. Cell. 2013;27:469–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

