FOXO transcription factors in cancer development and therapy
- PMID: 26686861
- PMCID: PMC11108379
- DOI: 10.1007/s00018-015-2112-y
FOXO transcription factors in cancer development and therapy
Abstract
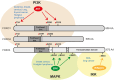
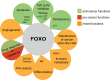
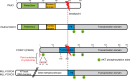
The forkhead box O (FOXO) transcription factors are considered as tumor suppressors that limit cell proliferation and induce apoptosis. FOXO gene alterations have been described in a limited number of human cancers, such as rhabdomyosarcoma, leukemia and lymphoma. In addition, FOXO proteins are inactivated by major oncogenic signals such as the phosphatidylinositol-3 kinase pathway and MAP kinases. Their expression is also repressed by micro-RNAs in multiple cancer types. FOXOs are mediators of the tumor response to various therapies. However, paradoxical roles of FOXOs in cancer progression were recently described. FOXOs contribute to the maintenance of leukemia-initiating cells in acute and chronic myeloid leukemia. These factors may also promote invasion and metastasis of subsets of colon and breast cancers. Resistance to treatment was also ascribed to FOXO activation in multiple cases, including targeted therapies. In this review, we discuss the complex role of FOXOs in cancer development and response to therapy.
Keywords: Cancer stem cells; Cell cycle; Cell invasion; FOXO1; FOXO3; FOXO4; Metastasis; Tumor-initiating cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

