Thrombospondins in the transition from myocardial infarction to heart failure
- PMID: 26686988
- PMCID: PMC4718798
- DOI: 10.1016/j.yjmcc.2015.12.009
Thrombospondins in the transition from myocardial infarction to heart failure
Abstract
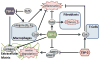
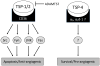
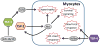
The heart's reaction to ischemic injury from a myocardial infarction involves complex cross-talk between the extra-cellular matrix (ECM) and different cell types within the myocardium. The ECM functions not only as a scaffold where myocytes beat synchronously, but an active signaling environment that regulates the important post-MI responses. The thrombospondins are matricellular proteins that modulate cell--ECM interactions, functioning as "sensors" that mediate outside-in and inside-out signaling. Thrombospondins are highly expressed during embryonic stages, and although their levels decrease during adult life, can be re-expressed in high quantities in response to cardiac stress including myocardial infarction and heart failure. Like a Swiss-army knife, the thrombospondins possess many tools: numerous binding domains that allow them to interact with other elements of the ECM, cell surface receptors, and signaling molecules. It is through these that the thrombospondins function. In the present review, we provide basic as well as clinical evidence linking the thrombospondin proteins with the post myocardial infarction response, including inflammation, fibrotic matrix remodeling, angiogenesis, as well as myocyte hypertrophy, apoptosis, and contractile dysfunction in heart failure. We will describe what is known regarding the intracellular signaling pathways that are involved with these responses, paving the road for future studies identifying these proteins as therapeutic targets for cardiac disease.
Keywords: Angiogenesis; Heart failure; Myocardial infarction; Remodeling; TGFβ; Thrombospondin.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
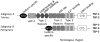
Similar articles
-
Extracellular matrix-mediated cellular communication in the heart.J Mol Cell Cardiol. 2016 Feb;91:228-37. doi: 10.1016/j.yjmcc.2016.01.011. Epub 2016 Jan 14. J Mol Cell Cardiol. 2016. PMID: 26778458 Free PMC article. Review.
-
T-cell regulation of fibroblasts and cardiac fibrosis.Matrix Biol. 2020 Sep;91-92:167-175. doi: 10.1016/j.matbio.2020.04.001. Epub 2020 May 11. Matrix Biol. 2020. PMID: 32438054 Free PMC article. Review.
-
Deficiency of Capn4 gene inhibits nuclear factor-κB (NF-κB) protein signaling/inflammation and reduces remodeling after myocardial infarction.J Biol Chem. 2012 Aug 10;287(33):27480-9. doi: 10.1074/jbc.M112.358929. Epub 2012 Jun 29. J Biol Chem. 2012. PMID: 22753411 Free PMC article.
-
Regenerative cross talk between cardiac cells and macrophages.Am J Physiol Heart Circ Physiol. 2021 Jun 1;320(6):H2211-H2221. doi: 10.1152/ajpheart.00056.2021. Epub 2021 Mar 26. Am J Physiol Heart Circ Physiol. 2021. PMID: 33769920 Free PMC article.
-
Regulators of cardiac fibroblast cell state.Matrix Biol. 2020 Sep;91-92:117-135. doi: 10.1016/j.matbio.2020.04.002. Epub 2020 May 19. Matrix Biol. 2020. PMID: 32416242 Free PMC article. Review.
Cited by
-
Altered expression of thrombospondin-1/-2 in the cortex and synaptophysin in the hippocampus after middle cerebral artery occlusion and reperfusion.Int J Clin Exp Pathol. 2018 Jul 1;11(7):3267-3276. eCollection 2018. Int J Clin Exp Pathol. 2018. PMID: 31949701 Free PMC article.
-
Extracellular matrix-mediated cellular communication in the heart.J Mol Cell Cardiol. 2016 Feb;91:228-37. doi: 10.1016/j.yjmcc.2016.01.011. Epub 2016 Jan 14. J Mol Cell Cardiol. 2016. PMID: 26778458 Free PMC article. Review.
-
Comparative mRNA and MicroRNA Profiling during Acute Myocardial Infarction Induced by Coronary Occlusion and Ablation Radio-Frequency Currents.Front Physiol. 2016 Nov 25;7:565. doi: 10.3389/fphys.2016.00565. eCollection 2016. Front Physiol. 2016. PMID: 27932994 Free PMC article.
-
Thrombospondin-2 holds prognostic value and is associated with metastasis and the mismatch repair process in gastric cancer.BMC Cancer. 2022 Mar 7;22(1):250. doi: 10.1186/s12885-022-09201-3. BMC Cancer. 2022. PMID: 35255858 Free PMC article.
-
Matrix metalloproteinases as input and output signals for post-myocardial infarction remodeling.J Mol Cell Cardiol. 2016 Feb;91:134-40. doi: 10.1016/j.yjmcc.2015.12.018. Epub 2015 Dec 23. J Mol Cell Cardiol. 2016. PMID: 26721597 Free PMC article. Review.
References
-
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–322. - PubMed
-
- Bradshaw AD. Macrophage Expression of Secreted Protein Acidic and Rich in Cysteine (SPARC): Implications for Cardiac Repair and Fibrosis. J Mol Cell Cardiol. 2016 in press, JMCC9593. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

