Antibiotic regimen based on population analysis of residing persister cells eradicates Staphylococcus epidermidis biofilms
- PMID: 26687035
- PMCID: PMC4685274
- DOI: 10.1038/srep18578
Antibiotic regimen based on population analysis of residing persister cells eradicates Staphylococcus epidermidis biofilms
Abstract
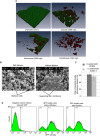
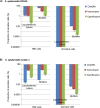
Biofilm formation is a major pathogenicity strategy of Staphylococcus epidermidis causing various medical-device infections. Persister cells have been implicated in treatment failure of such infections. We sought to profile bacterial subpopulations residing in S. epidermidis biofilms, and to establish persister-targeting treatment strategies to eradicate biofilms. Population analysis was performed by challenging single biofilm cells with antibiotics at increasing concentrations ranging from planktonic minimum bactericidal concentrations (MBCs) to biofilm MBCs (MBCbiofilm). Two populations of "persister cells" were observed: bacteria that survived antibiotics at MBCbiofilm for 24/48 hours were referred to as dormant cells; those selected with antibiotics at 8 X MICs for 3 hours (excluding dormant cells) were defined as tolerant-but-killable (TBK) cells. Antibiotic regimens targeting dormant cells were tested in vitro for their efficacies in eradicating persister cells and intact biofilms. This study confirmed that there are at least three subpopulations within a S. epidermidis biofilm: normal cells, dormant cells, and TBK cells. Biofilms comprise more TBK cells and dormant cells than their log-planktonic counterparts. Using antibiotic regimens targeting dormant cells, i.e. effective antibiotics at MBCbiofilm for an extended period, might eradicate S. epidermidis biofilms. Potential uses for this strategy are in antibiotic lock techniques and inhaled aerosolized antibiotics.
Figures


References
-
- Percival S. L., Hill K. E., Malic S., Thomas D. W. & Williams D. W. Antimicrobial tolerance and the significance of persister cells in recalcitrant chronic wound biofilms. Wound Repair Regen. 19, 1–9 (2011). - PubMed
-
- Roberts M. E. & Stewart P. S. Modelling protection from antimicrobial agents in biofilms through the formation of persister cells. Microbiology 151, 75–80 (2005). - PubMed
-
- Lewis K. Persister cells and the riddle of biofilm survival. Biochemistry (Mosc) 70, 267–274 (2005). - PubMed
-
- Lewis K. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5, 48–56 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

