bFGF Protects Against Blood-Brain Barrier Damage Through Junction Protein Regulation via PI3K-Akt-Rac1 Pathway Following Traumatic Brain Injury
- PMID: 26687235
- PMCID: PMC5516728
- DOI: 10.1007/s12035-015-9583-6
bFGF Protects Against Blood-Brain Barrier Damage Through Junction Protein Regulation via PI3K-Akt-Rac1 Pathway Following Traumatic Brain Injury
Abstract
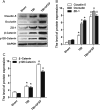
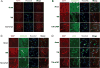
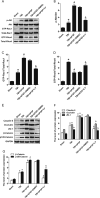
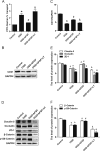
Many traumatic brain injury (TBI) survivors sustain neurological disability and cognitive impairments due to the lack of defined therapies to reduce TBI-induced blood-brain barrier (BBB) breakdown. Exogenous basic fibroblast growth factor (bFGF) has been shown to have neuroprotective function in brain injury. The present study therefore investigates the beneficial effects of bFGF on the BBB after TBI and the underlying mechanisms. In this study, we demonstrate that bFGF reduces neurofunctional deficits and preserves BBB integrity in a mouse model of TBI. bFGF suppresses RhoA and upregulates tight junction proteins, thereby mitigating BBB breakdown. In vitro, bFGF exerts a protective effect on BBB by upregulating tight junction proteins claudin-5, occludin, zonula occludens-1, p120-catenin, and β-catenin under oxygen glucose deprivation/reoxygenation (OGD) in human brain microvascular endothelial cells (HBMECs). Both the in vivo and in vitro effects are related to the activation of the downstream signaling pathway, PI3K/Akt/Rac-1. Inhibition of the PI3K/Akt or Rac-1 by specific inhibitors LY294002 or si-Rac-1, respectively, partially reduces the protective effect of bFGF on BBB integrity. Overall, our results indicate that the protective role of bFGF on BBB involves the regulation of tight junction proteins and RhoA in the TBI model and OGD-induced HBMECs injury, and that activation of the PI3K/Akt /Rac-1 signaling pathway underlies these effects.
Keywords: Blood-brain barrier; PI3K/Akt /Rac-1; RhoA; Tight junction; Traumatic brain injury; bFGF.
Figures





References
-
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD. Surveillance for traumatic brain injury-related deaths—United States, 1997-2007. MMWR Surveill Summ. 2011;60(5):1–32. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

