Glycoproteomic studies of IgE from a novel hyper IgE syndrome linked to PGM3 mutation
- PMID: 26687240
- PMCID: PMC4891365
- DOI: 10.1007/s10719-015-9638-y
Glycoproteomic studies of IgE from a novel hyper IgE syndrome linked to PGM3 mutation
Abstract
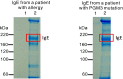
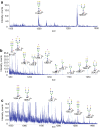
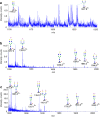
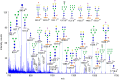
Glycans serve as important regulators of antibody activities and half-lives. IgE is the most heavily glycosylated antibody, but in comparison to other antibodies little is known about its glycan structure function relationships. We therefore describe the site specific IgE glycosylation from a patient with a novel hyper IgE syndrome linked to mutations in PGM3, which is an enzyme involved in synthesizing UDP-GlcNAc, a sugar donor widely required for glycosylation. A two-step method was developed to prepare two IgE samples from less than 1 mL of serum collected from a patient with PGM3 mutation and a patient with atopic dermatitis as a control subject. Then, a glycoproteomic strategy was used to study the site-specific glycosylation. No glycosylation was found at Asn264, whilst high mannose glycans were only detected at Asn275, tri-antennary glycans were exclusively observed at Asn99 and Asn252, and non-fucosylated complex glycans were detected at Asn99. The results showed similar glycosylation profiles between the two IgE samples. These observations, together with previous knowledge of IgE glycosylation, imply that IgE glycosylation is similarly regulated among healthy control, allergy and PGM3 related hyper IgE syndrome.
Keywords: Glycoproteomics; Hyper IgE Syndrome; IgE; Phosphoglucomutase 3.
Figures

References
-
- Parekh RB, Dwek RA, Sutton BJ, Fernandes DL, Leung A, Stanworth D, Rademacher TW, Mizuochi T, Taniguchi T, Matsuta K, et al. Association of rheumatoid arthritis and primary osteoarthritis with changes in the glycosylation pattern of total serum IgG. Nature. 1985;316(6027):452–457. doi: 10.1038/316452a0. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

