Genetic Identification of an Expansive Mechanoreceptor Sensitive to Skin Stroking
- PMID: 26687362
- PMCID: PMC4890169
- DOI: 10.1016/j.cell.2015.11.060
Genetic Identification of an Expansive Mechanoreceptor Sensitive to Skin Stroking
Abstract
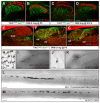
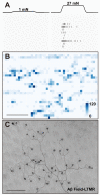
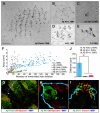
Touch perception begins with activation of low-threshold mechanoreceptors (LTMRs) in the periphery. LTMR terminals exhibit tremendous morphological heterogeneity that specifies their mechanical receptivity. In a survey of mammalian skin, we found a preponderance of neurofilament-heavy-chain(+) circumferential endings associated with hair follicles, prompting us to develop a genetic strategy to interrogate these neurons. Targeted in vivo recordings revealed them to be Aβ field-LTMRs, identified 50 years ago but largely elusive thereafter. Remarkably, while Aβ field-LTMRs are highly sensitive to gentle stroking of the skin, they are unresponsive to hair deflection, and they encode skin indentation in the noxious range across large, spotty receptive fields. Individual Aβ field-LTMRs form up to 180 circumferential endings, making them the most anatomically expansive LTMR identified to date. Thus, Aβ field-LTMRs are a major mammalian LTMR subtype that forms circumferential endings in hairy skin, and their sensitivity to gentle skin stroking arises through integration across many low-sensitivity circumferential endings.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




References
-
- Ballermann M, McKenna J, Whishaw IQ. A grasp-related deficit in tactile discrimination following dorsal column lesion in the rat. Brain Res. Bull. 2001;54:237–242. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

