SPED Light Sheet Microscopy: Fast Mapping of Biological System Structure and Function
- PMID: 26687363
- PMCID: PMC4775738
- DOI: 10.1016/j.cell.2015.11.061
SPED Light Sheet Microscopy: Fast Mapping of Biological System Structure and Function
Abstract
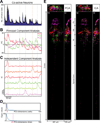
The goal of understanding living nervous systems has driven interest in high-speed and large field-of-view volumetric imaging at cellular resolution. Light sheet microscopy approaches have emerged for cellular-resolution functional brain imaging in small organisms such as larval zebrafish, but remain fundamentally limited in speed. Here, we have developed SPED light sheet microscopy, which combines large volumetric field-of-view via an extended depth of field with the optical sectioning of light sheet microscopy, thereby eliminating the need to physically scan detection objectives for volumetric imaging. SPED enables scanning of thousands of volumes-per-second, limited only by camera acquisition rate, through the harnessing of optical mechanisms that normally result in unwanted spherical aberrations. We demonstrate capabilities of SPED microscopy by performing fast sub-cellular resolution imaging of CLARITY mouse brains and cellular-resolution volumetric Ca(2+) imaging of entire zebrafish nervous systems. Together, SPED light sheet methods enable high-speed cellular-resolution volumetric mapping of biological system structure and function.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






References
-
- Ahrens MB, Orger MB, Robson DN, Li JM, Keller PJ. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Methods. 2013;10:413–420. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

