A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study
- PMID: 26687425
- PMCID: PMC4340092
- DOI: 10.1016/S2352-3026(14)00035-0
A prognostic score for acute graft-versus-host disease based on biomarkers: a multicentre study
Abstract
Background: Graft-versus-host disease (GVHD) is the major cause of non-relapse mortality after allogeneic haemopoietic stem-cell transplantation (SCT). The severity of symptoms at the onset of GVHD does not accurately define risk, and thus most patients are treated alike with high dose systemic corticosteroids. We aimed to define clinically meaningful risk strata for patients with newly diagnosed acute GVHD using plasma biomarkers.
Methods: Between April 13, 2000, and May 7, 2013, we prospectively collected plasma from 492 SCT patients with newly diagnosed acute GVHD and randomly assigned (2:1) using a random number generator, conditional on the final two datasets having the same median day of onset, into training (n=328) and test (n=164) sets. We used the concentrations of three recently validated biomarkers (TNFR1, ST2, and Reg3α) to create an algorithm that computed the probability of non-relapse mortality 6 months after GVHD onset for individual patients in the training set alone. We rank ordered the probabilities and identified thresholds that created three distinct non-relapse mortality scores. We evaluated the algorithm in the test set, and again in an independent validation set of an additional 300 patients who underwent stem cell transplant and were enrolled on multicentre clinical trials of primary therapy for acute GVHD.
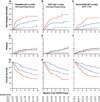
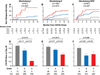
Findings: In all three datasets (training, test, and validation), the cumulative incidence of 6-month non-relapse mortality significantly increased as the Ann Arbor GVHD score increased. In the multicentre validation set, scores were 8% (95% CI 3-16) for score 1, 27% (20-34) for score 2, and 46% (33-58) for score 3 (p<0·0001). Conversely, the response to primary GVHD treatment within 28 days decreased as the GVHD score increased 86% for score 1, 67% for score 2, and 46% for score 3 in the multicentre validation set, p<0·0001).
Interpretation: Biomarker-based scores can be used to guide risk-adapted therapy at the onset of acute GVHD. High risk patients with a score of 3 are candidates for intensive primary therapy, while low risk patients with a score of 1 are candidates for rapid tapers of systemic steroid therapy.
Funding: The National Cancer Institute, the National Heart, Lung, and Blood Institute, the National Institute of Allergy and Infectious Diseases, the Doris Duke Charitable Fund, the American Cancer Society, and the Judith Devries Fund.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures


Comment in
-
Biomarkers for GVHD prognosis.Lancet Haematol. 2015 Jan;2(1):e4-5. doi: 10.1016/S2352-3026(14)00040-4. Epub 2014 Dec 23. Lancet Haematol. 2015. PMID: 26687427 No abstract available.
References
-
- Socie G, Ritz J, Martin PJ. Current challenges in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16:S146–S151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U10 HL069294/HL/NHLBI NIH HHS/United States
- P30 CA016672/CA/NCI NIH HHS/United States
- R21 CA173459/CA/NCI NIH HHS/United States
- P30 CA046592/CA/NCI NIH HHS/United States
- U10 HL069290/HL/NHLBI NIH HHS/United States
- U24 CA076518/CA/NCI NIH HHS/United States
- U10 HL069330/HL/NHLBI NIH HHS/United States
- P01CA039542/CA/NCI NIH HHS/United States
- U10 HL069249/HL/NHLBI NIH HHS/United States
- U10 HL069334/HL/NHLBI NIH HHS/United States
- U10 HL069310/HL/NHLBI NIH HHS/United States
- U10 HL109137/HL/NHLBI NIH HHS/United States
- P30CA046592/CA/NCI NIH HHS/United States
- U10HL069294/HL/NHLBI NIH HHS/United States
- U01 HL069330/HL/NHLBI NIH HHS/United States
- P01 CA039542/CA/NCI NIH HHS/United States
- R21CA173459/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

