Thrombopoietin induces production of nucleated thrombocytes from liver cells in Xenopus laevis
- PMID: 26687619
- PMCID: PMC4685256
- DOI: 10.1038/srep18519
Thrombopoietin induces production of nucleated thrombocytes from liver cells in Xenopus laevis
Abstract
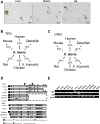
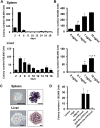
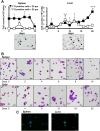
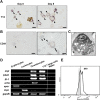
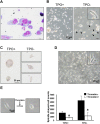
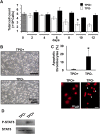
The development of mammalian megakaryocytes (MKs) and platelets, which are thought to be absent in non-mammals, is primarily regulated by the thrombopoietin (TPO)/Mpl system. Although non-mammals possess nucleated thrombocytes instead of platelets, the features of nucleated thrombocyte progenitors remain to be clarified. Here, we provide the general features of TPO using Xenopus laevis TPO (xlTPO). Hepatic and splenic cells were cultured in liquid suspension with recombinant xlTPO. These cells differentiated into large, round, polyploid CD41-expressing cells and were classified as X. laevis MKs, comparable to mammalian MKs. The subsequent culture of MKs after removal of xlTPO produced mature, spindle-shaped thrombocytes that were activated by thrombin, thereby altering their morphology. XlTPO induced MKs in cultured hepatic cells for at least three weeks; however, this was not observed in splenic cells; this result demonstrates the origin of early haematopoietic progenitors in the liver rather than the spleen. Additionally, xlTPO enhanced viability of peripheral thrombocytes, indicating the xlTPO-Mpl pathway stimulates anti-apoptotic in peripheral thrombocytes. The development of thrombocytes from MKs via the TPO-Mpl system in X. laevis plays a crucial role in their development from MKs, comparable to mammalian thrombopoiesis. Thus, our results offer insight into the cellular evolution of platelets/MKs in vertebrates. (200/200).
Figures
Similar articles
-
Cellular characterization of thrombocytes in Xenopus laevis with specific monoclonal antibodies.Exp Hematol. 2015 Feb;43(2):125-36. doi: 10.1016/j.exphem.2014.10.005. Epub 2014 Oct 22. Exp Hematol. 2015. PMID: 25448492
-
Thrombopoietin (TPO) induces thrombocytic colony formation of kidney cells synergistically with kit ligand A and a non-secretory TPO variant exists in common carp.Dev Comp Immunol. 2018 Jul;84:327-336. doi: 10.1016/j.dci.2018.03.005. Epub 2018 Mar 6. Dev Comp Immunol. 2018. PMID: 29522790
-
OP9 bone marrow stroma cells differentiate into megakaryocytes and platelets.PLoS One. 2013;8(3):e58123. doi: 10.1371/journal.pone.0058123. Epub 2013 Mar 1. PLoS One. 2013. PMID: 23469264 Free PMC article.
-
Advances in megakaryocytopoiesis and thrombopoiesis: from bench to bedside.Br J Haematol. 2013 Jun;161(6):778-93. doi: 10.1111/bjh.12328. Epub 2013 Apr 18. Br J Haematol. 2013. PMID: 23594368 Review.
-
Milestones in understanding platelet production: a historical overview.Br J Haematol. 2014 Apr;165(2):248-58. doi: 10.1111/bjh.12781. Epub 2014 Feb 14. Br J Haematol. 2014. PMID: 24528208 Review.
Cited by
-
Flow cytometric analysis of Xenopus laevis and X. tropicalis blood cells using acridine orange.Sci Rep. 2018 Nov 2;8(1):16245. doi: 10.1038/s41598-018-34631-0. Sci Rep. 2018. PMID: 30390005 Free PMC article.
References
-
- Bentfeld-Barker M. E. & Bainton D. F. Identification of primary lysosomes in human megakaryocytes and platelets. Blood 59, 472–481 (1982). - PubMed
-
- Souyri M. et al. A putative truncated cytokine receptor gene transduced by the myeloproliferative leukemia virus immortalizes hematopoietic progenitors. Cell 63, 1137–1147 (1990). - PubMed
-
- Methia N., Louache F., Vainchenker W. & Wendling F. Oligodeoxynucleotides antisense to the proto-oncogene c-mpl specifically inhibit in vitro megakaryocytopoiesis. Blood 82, 1395–1401 (1993). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

