Cell-Specific Transcriptional Profiling of Ciliated Sensory Neurons Reveals Regulators of Behavior and Extracellular Vesicle Biogenesis
- PMID: 26687621
- PMCID: PMC4698341
- DOI: 10.1016/j.cub.2015.10.057
Cell-Specific Transcriptional Profiling of Ciliated Sensory Neurons Reveals Regulators of Behavior and Extracellular Vesicle Biogenesis
Abstract
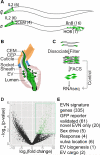
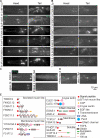
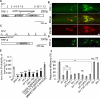
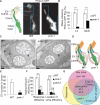
Cilia and extracellular vesicles (EVs) are signaling organelles [1]. Cilia act as cellular sensory antennae, with defects resulting in human ciliopathies. Cilia both release and bind to EVs [1]. EVs are sub-micron-sized particles released by cells and function in both short- and long-range intercellular communication. In C. elegans and mammals, the autosomal dominant polycystic kidney disease (ADPKD) gene products polycystin-1 and polycystin-2 localize to both cilia and EVs, act in the same genetic pathway, and function in a sensory capacity, suggesting ancient conservation [2]. A fundamental understanding of EV biology and the relationship between the polycystins, cilia, and EVs is lacking. To define properties of a ciliated EV-releasing cell, we performed RNA-seq on 27 GFP-labeled EV-releasing neurons (EVNs) isolated from adult C. elegans. We identified 335 significantly overrepresented genes, of which 61 were validated by GFP reporters. The EVN transcriptional profile uncovered new pathways controlling EV biogenesis and polycystin signaling and also identified EV cargo, which included an antimicrobial peptide and ASIC channel. Tumor-necrosis-associated factor (TRAF) homologs trf-1 and trf-2 and the p38 mitogen-activated protein kinase (MAPK) pmk-1 acted in polycystin-signaling pathways controlling male mating behaviors. pmk-1 was also required for EV biogenesis, independent of the innate immunity MAPK signaling cascade. This first high-resolution transcriptome profile of a subtype of ciliated sensory neurons isolated from adult animals reveals the functional components of an EVN.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

