Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects
- PMID: 26687841
- PMCID: PMC4754122
- DOI: 10.1016/j.neuron.2015.11.023
Mice with Shank3 Mutations Associated with ASD and Schizophrenia Display Both Shared and Distinct Defects
Abstract
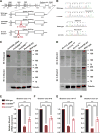
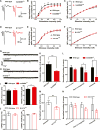
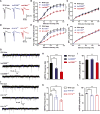
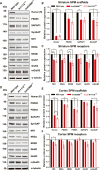
Genetic studies have revealed significant overlaps of risk genes among psychiatric disorders. However, it is not clear how different mutations of the same gene contribute to different disorders. We characterized two lines of mutant mice with Shank3 mutations linked to ASD and schizophrenia. We found both shared and distinct synaptic and behavioral phenotypes. Mice with the ASD-linked InsG3680 mutation manifest striatal synaptic transmission defects before weaning age and impaired juvenile social interaction, coinciding with the early onset of ASD symptoms. On the other hand, adult mice carrying the schizophrenia-linked R1117X mutation show profound synaptic defects in prefrontal cortex and social dominance behavior. Furthermore, we found differential Shank3 mRNA stability and SHANK1/2 upregulation in these two lines. These data demonstrate that different alleles of the same gene may have distinct phenotypes at molecular, synaptic, and circuit levels in mice, which may inform exploration of these relationships in human patients.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures



Comment in
-
News Feature: Better models for brain disease.Proc Natl Acad Sci U S A. 2016 May 17;113(20):5461-4. doi: 10.1073/pnas.1605358113. Proc Natl Acad Sci U S A. 2016. PMID: 27190074 Free PMC article. No abstract available.
References
-
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7:268–277. - PubMed
-
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nat Neurosci. 1999;2:1032–1037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

