Fast virtual histology using X-ray in-line phase tomography: application to the 3D anatomy of maize developing seeds
- PMID: 26688690
- PMCID: PMC4684619
- DOI: 10.1186/s13007-015-0098-y
Fast virtual histology using X-ray in-line phase tomography: application to the 3D anatomy of maize developing seeds
Abstract
Background: Despite increasing demand, imaging the internal structure of plant organs or tissues without the use of transgenic lines expressing fluorescent proteins remains a challenge. Techniques such as magnetic resonance imaging, optical projection tomography or X-ray absorption tomography have been used with various success, depending on the size and physical properties of the biological material.
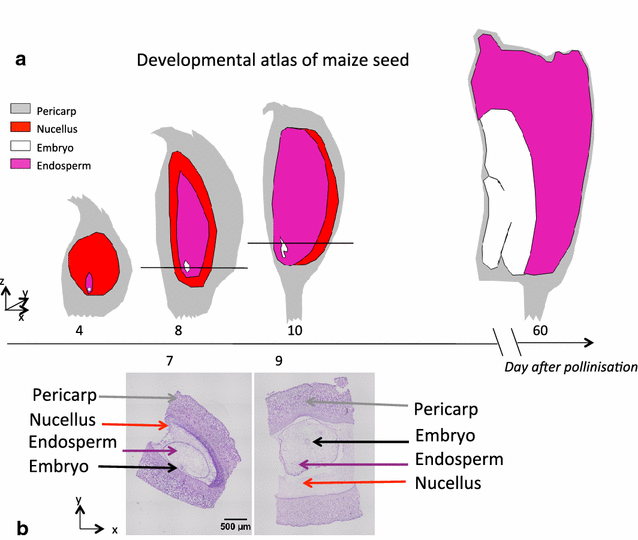
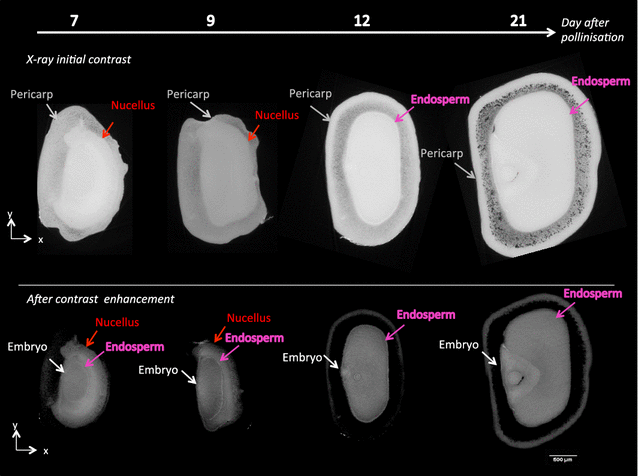
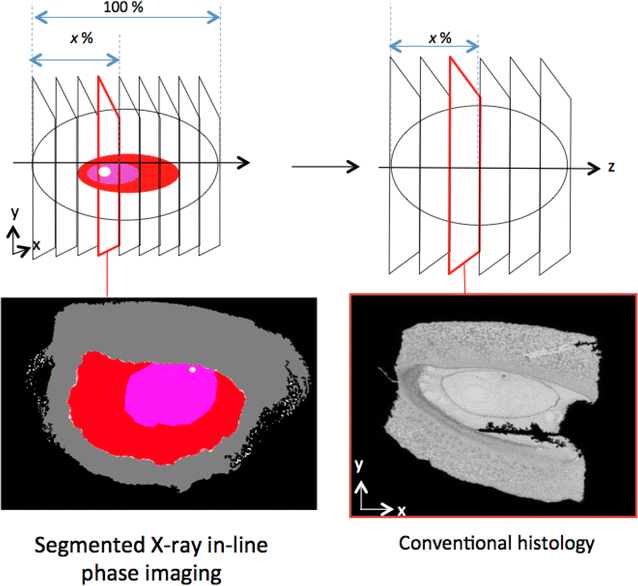
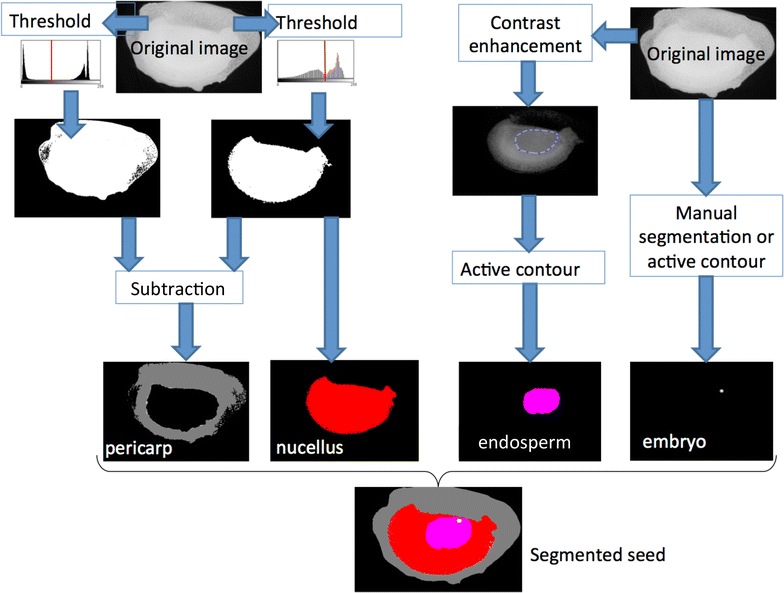
Results: X-ray in-line phase tomography was applied for the imaging of internal structures of maize seeds at early stages of development, when the cells are metabolically fully active and water is the main cell content. This 3D imaging technique with histology-like spatial resolution is demonstrated to reveal the anatomy of seed compartments with unequalled contrast by comparison with X-ray absorption tomography. An associated image processing pipeline allowed to quantitatively segment in 3D the four compartments of the seed (embryo, endosperm, nucellus and pericarp) from 7 to 21 days after pollination.
Conclusion: This work constitutes an innovative quantitative use of X-ray in-line phase tomography as a non-destructive fast method to perform virtual histology and extends the developmental stages accessible by this technique which had previously been applied in seed biology to more mature samples.
Keywords: Image segmentation; Virtual histology; X ray in-line phase tomography; maize seeds; plant development.
Figures


References
-
- Metzner R, Eggert A, van Dusschoten D, Pflugfelder D, Gerth S, Schurr U, Uhlmann N, Jahnke S. Direct comparison of mri and X-ray CT technologies for 3D imaging of root systems in soil: potential and challenges for root trait quantification. Plant Methods. 2015;11(1):17. doi: 10.1186/s13007-015-0060-z. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

