Ciprofloxacin DPI: a randomised, placebo-controlled, phase IIb efficacy and safety study on cystic fibrosis
- PMID: 26688732
- PMCID: PMC4680008
- DOI: 10.1136/bmjresp-2015-000100
Ciprofloxacin DPI: a randomised, placebo-controlled, phase IIb efficacy and safety study on cystic fibrosis
Abstract
Background: Treatment of infective bronchitis involving Pseudomonas aeruginosa is a cornerstone of care in patients with cystic fibrosis (CF). This phase IIb, randomised, double-blind, placebo-controlled study assessed the efficacy and safety of ciprofloxacin dry powder for inhalation (DPI) in this population.
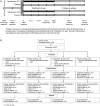
Methods: Patients with CF, ≥12 years of age (N=286), were randomised to ciprofloxacin DPI (32.5 mg (n=93) or 48.75 mg (n=93)), or corresponding placebo (32.5 mg, n=65; 48.75 mg, n=35) twice daily for 28 days. The primary objective was the change in forced expiratory volume in 1 s (FEV1) from baseline (day 0) to end of treatment (day 29) in the intent-to-treat population for ciprofloxacin DPI compared with the corresponding placebo group.
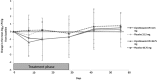
Results: The primary effectiveness objective was not met; there were no significant differences in change in FEV1 between ciprofloxacin DPI and the corresponding placebo group for either dose (p=0.154). However, in pooled analyses, FEV1 decline from baseline to treatment end was significantly lower with ciprofloxacin DPI than with placebo (pooled data; p=0.02). Ciprofloxacin DPI showed positive effects on sputum bacterial load and quality of life, but these effects were not maintained at the 4-week follow-up. Ciprofloxacin DPI was well tolerated and there were no significant differences in type/incidence of treatment-emergent adverse events by treatment group (p=0.115).
Conclusions: Further investigations are needed to determine the full scope of the beneficial effects of ciprofloxacin DPI for patients with CF.
Trial registration number: Clinicaltrials.gov NCT00645788; EudraCT 2008-008314-40.
Keywords: Cystic Fibrosis.
Figures

References
-
- Gibson RL, Burns JL, Ramsey BW. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am J Respir Crit Care Med 2003;168:918–51. doi:10.1164/rccm.200304-505SO - DOI - PubMed
-
- Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med 2006;173:475–82. doi:10.1164/rccm.200505-840OE - DOI - PubMed
-
- Cystic Fibrosis Foundation 2011 Patient Registry Report Cystic Fibrosis Foundation, 2011. http://www.cff.org/UploadedFiles/research/ClinicalResearch/2011-Patient-... (accessed Mar 2015).
-
- Flume PA, Robinson KA, O'Sullivan BP et al. . Cystic fibrosis pulmonary guidelines: airway clearance therapies. Respir Care 2009;54:522–37. - PubMed
-
- Bendiak GN, Ratjen F. The approach to Pseudomonas aeruginosa in cystic fibrosis. Semin Respir Crit Care Med 2009;30:587–95. doi:10.1055/s-0029-1238917 - DOI - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
