The Yin and Yang of Chromatin Dynamics In Stem Cell Fate Selection
- PMID: 26689127
- PMCID: PMC4733603
- DOI: 10.1016/j.tig.2015.11.002
The Yin and Yang of Chromatin Dynamics In Stem Cell Fate Selection
Abstract
Adult organisms rely on tissue stem cells for maintenance and repair. During homeostasis, the concerted action of local niche signals and epigenetic regulators establish stable gene expression patterns to ensure that stem cells are not lost over time. However, stem cells also provide host tissues with a remarkable plasticity to respond to perturbations. How adult stem cells choose and acquire new fates is unknown, but the genome-wide mapping of epigenetic landscapes suggests a critical role for chromatin remodeling in these processes. Here, we explore the emerging role of chromatin modifiers and pioneer transcription factors in adult stem cell fate decisions and plasticity, which ensure that selective lineage choices are only made when environmentally cued.
Keywords: adult stem cells; cell identity; chromatin dynamics; fate selection; plasticity; super-enhancer.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Figures
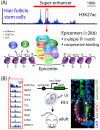
References
-
- Bergman Y, Cedar H. DNA methylation dynamics in health and disease. Nat. Struct. Mol. Biol. 2013;20:274–281. - PubMed
-
- Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–220. - PubMed
-
- Sharif J, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–912. - PubMed
-
- Schübeler D. Function and information content of DNA methylation. Nature. 2015;517:321–326. - PubMed
-
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

