Cell-Permeable Esterase-Activated Ca(II)-Sensitive MRI Contrast Agent
- PMID: 26689452
- PMCID: PMC4776747
- DOI: 10.1021/acs.bioconjchem.5b00561
Cell-Permeable Esterase-Activated Ca(II)-Sensitive MRI Contrast Agent
Abstract
Calcium [Ca(II)] is a fundamental transducer of electrical activity in the central nervous system (CNS). Influx of Ca(II) into the cytosol is responsible for action potential initiation and propagation, and initiates interneuronal communication via release of neurotransmitters and activation of gene expression. Despite the importance of Ca(II) in physiology, it remains a challenge to visualize Ca(II) flux in the central nervous system (CNS) in vivo. To address these challenges, we have developed a new generation, Ca(II)-activated MRI contrast agent that utilizes ethyl esters to increase cell labeling and prevent extracellular divalent Ca(II) binding. Following labeling, the ethyl esters can be cleaved, thus allowing the agent to bind Ca(II), increasing relaxivity and resulting in enhanced positive MR image contrast. The ability of this probe to discriminate between extra- and intracellular Ca(II) may allow for spatiotemporal in vivo imaging of Ca(II) flux during seizures or ischemia where large Ca(II) fluxes (1-10 μM) can result in cell death.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
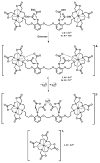

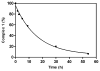
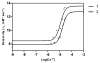



References
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Bading H. Nuclear calcium signalling in the regulation of brain function. Nat Rev Neurosci. 2013;14:593–608. - PubMed
-
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. - PubMed
-
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. - PubMed
-
- Charles A. Intercellular calcium waves in glia. Glia. 1998;24:39–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

