Exploring the cause of initially reactive bovine brains on rapid tests for BSE
- PMID: 26689488
- PMCID: PMC4964865
- DOI: 10.1080/19336896.2015.1115945
Exploring the cause of initially reactive bovine brains on rapid tests for BSE
Abstract
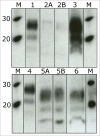
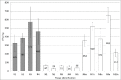
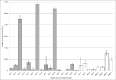
Bovine spongiform encephalopathy (BSE) is an invariably fatal prion disease of cattle. The identification of the zoonotic potential of BSE prompted safety officials to initiate surveillance testing for this disease. In Canada, BSE surveillance is primarily focused on high risk cattle including animals which are dead, down and unable to rise, diseased or distressed. This targeted surveillance results in the submission of brain samples with a wide range of tissue autolysis and associated contaminants. These contaminants have the potential to interfere with important steps of surveillance tests resulting in initially positive test results requiring additional testing to confirm the disease status of the animal. The current tests used for BSE screening in Canada utilize the relative protease resistance of the prion protein gained when it misfolds from PrP(C) to PrP(Sc) as part of the disease process. Proteinase K completely digests PrP(C) in normal brains, but leaves most of the PrP(Sc) in BSE positive brains intact which is detected using anti-prion antibodies. These tests are highly reliable but occasionally give rise to initially reactive/false positive results. Test results for these reactive samples were close to the positive/negative cut-off on a sub set of test platforms. This is in contrast to all of the previous Canadian positive samples whose numeric values on these same test platforms were 10 to 100 fold greater than the test positive/negative cut-off. Here we explore the potential reason why a sample is repeatedly positive on a sub-set of rapid surveillance tests, but negative on other test platforms. In order to better understand and identify what might cause these initial reactions, we have conducted a variety of rapid and confirmatory assays as well as bacterial isolation and identification on BSE positive, negative and initially reactive samples. We observed high levels of viable bacterial contamination in initially reactive samples suggesting that the reactivity may be related to bacterial factors. Several bacteria isolated from the initially reactive samples have characteristics of biofilm forming bacteria and this extracellular matrix might play a role in preventing complete digestion of PrP(C) in these samples.
Keywords: bacterial contamination;; bovine spongiform encephalopathy;; confirmatory testing;; diagnostics;; false positive; rapid testing,.
Figures

Similar articles
-
Atypical BSE: Current Knowledge and Knowledge Gaps.Food Saf (Tokyo). 2017 Feb 8;5(1):10-13. doi: 10.14252/foodsafetyfscj.2016028. eCollection 2017 Mar. Food Saf (Tokyo). 2017. PMID: 32231923 Free PMC article. Review.
-
Experimental transmission of two young and one suspended bovine spongiform encephalopathy (BSE) cases to bovinized transgenic mice.Jpn J Infect Dis. 2007 Sep;60(5):317-20. Jpn J Infect Dis. 2007. PMID: 17881878
-
Accumulation of mono-glycosylated form-rich, plaque-forming PrPSc in the second atypical bovine spongiform encephalopathy case in Japan.Jpn J Infect Dis. 2007 Sep;60(5):305-8. Jpn J Infect Dis. 2007. PMID: 17881874
-
PrP-C1 fragment in cattle brains reveals features of the transmissible spongiform encephalopathy associated PrPsc.Brain Res. 2017 Mar 15;1659:19-28. doi: 10.1016/j.brainres.2017.01.015. Epub 2017 Jan 22. Brain Res. 2017. PMID: 28119056
-
Bovine spongiform encephalopathy (BSE): the end of the beginning or the beginning of the end?Folia Neuropathol. 2004;42 Suppl A:55-68. Folia Neuropathol. 2004. PMID: 15449460 Review.
Cited by
-
Conventional and State-of-the-Art Detection Methods of Bovine Spongiform Encephalopathy (BSE).Int J Mol Sci. 2023 Apr 12;24(8):7135. doi: 10.3390/ijms24087135. Int J Mol Sci. 2023. PMID: 37108297 Free PMC article. Review.
-
Atypical BSE: Current Knowledge and Knowledge Gaps.Food Saf (Tokyo). 2017 Feb 8;5(1):10-13. doi: 10.14252/foodsafetyfscj.2016028. eCollection 2017 Mar. Food Saf (Tokyo). 2017. PMID: 32231923 Free PMC article. Review.
References
-
- Wells GA, Scott AC, Johnson CT, Gunning RF, Hancock RD, Jeffrey M, Dawson M, Bradley R. A novel progressive spongiform encephalopathy in cattle. Vet Rec 1987; 121(18):419-20; PMID:3424605; http://dx.doi.org/10.1136/vr.121.18.419 - DOI - PubMed
-
- Bruce ME, Will RG, Ironside JW, McConnell I, Drummond D, Suttie A, McCardle L, Chree A, Hope J, Birkett C, et al. . Transmissions to mice indicate that 'new variant' CJD is caused by the BSE agent. Nature 1997; 389(6650):498-501; PMID:9333239; http://dx.doi.org/10.1038/39057 - DOI - PubMed
-
- Canadian Food Inspection Agency Canada's protocols for BSE testing. 2012, December 27th Available at: http://www.inspection.gc.ca/animals/terrestrial-animals/diseases/reporta...
-
- Prusiner SB, Gabizon R, McKinley MP. On the biology of prion. Acta Neuropathol 1987; 72(4):299-314; PMID:3554880; http://dx.doi.org/10.1007/BF00687261 - DOI - PubMed
-
- Prusiner SB. Novel proteinaceous infectious part i cles cause scrapie. Science 1982; 216:136-44; PMID:6801762; http://dx.doi.org/10.1126/science.6801762 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
