KCa3.1 K+ Channel Expression and Function in Human Bronchial Epithelial Cells
- PMID: 26689552
- PMCID: PMC4687003
- DOI: 10.1371/journal.pone.0145259
KCa3.1 K+ Channel Expression and Function in Human Bronchial Epithelial Cells
Abstract
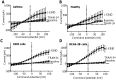
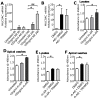
The KCa3.1 K+ channel has been proposed as a novel target for pulmonary diseases such as asthma and pulmonary fibrosis. It is expressed in epithelia but its expression and function in primary human bronchial epithelial cells (HBECs) has not been described. Due to its proposed roles in the regulation of cell proliferation, migration, and epithelial fluid secretion, inhibiting this channel might have either beneficial or adverse effects on HBEC function. The aim of this study was to assess whether primary HBECs express the KCa3.1 channel and its role in HBEC function. Primary HBECs from the airways of healthy and asthmatic subjects, SV-transformed BEAS-2B cells and the neoplastic H292 epithelial cell line were studied. Primary HBECs, BEAS-2B and H292 cells expressed KCa3.1 mRNA and protein, and robust KCa3.1 ion currents. KCa3.1 protein expression was increased in asthmatic compared to healthy airway epithelium in situ, and KCa3.1 currents were larger in asthmatic compared to healthy HBECs cultured in vitro. Selective KCa3.1 blockers (TRAM-34, ICA-17043) had no effect on epithelial cell proliferation, wound closure, ciliary beat frequency, or mucus secretion. However, several features of TGFβ1-dependent epithelial-mesenchymal transition (EMT) were inhibited by KCa3.1 blockade. Treatment with KCa3.1 blockers is likely to be safe with respect to airway epithelial biology, and may potentially inhibit airway remodelling through the inhibition of EMT.
Conflict of interest statement
Figures




References
-
- Asher MI, Montefort S, Bjorksten B, Lai CK, Strachan DP, Weiland SK et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006. 368: 733–743. - PubMed
-
- Duffy SM, Lawley WJ, Kaur D, Yang W, Bradding P. Inhibition of human mast cell proliferation and survival by tamoxifen in association with ion channel modulation. J Allergy Clin Immunol. 2003. 112: 970–977. - PubMed
-
- Tharp DL, Wamhoff BR, Turk JR, Bowles DK. Upregulation of intermediate-conductance Ca2+-activated K+ channel (IKCa1) mediates phenotypic modulation of coronary smooth muscle. Am J Physiol Heart Circ Physiol. 2006. 291: H2493–H2503. - PubMed
-
- Duffy SM, Lawley WJ, Conley EC, Bradding P. Resting and activation-dependent ion channels in human mast cells. J Immunol. 2001. 167: 4261–4270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

