Nicotine induces self-renewal of pancreatic cancer stem cells via neurotransmitter-driven activation of sonic hedgehog signalling
- PMID: 26689865
- PMCID: PMC4698183
- DOI: 10.1016/j.ejca.2015.10.003
Nicotine induces self-renewal of pancreatic cancer stem cells via neurotransmitter-driven activation of sonic hedgehog signalling
Abstract
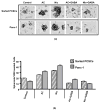
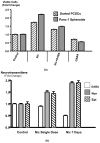
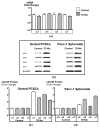
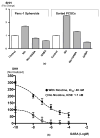
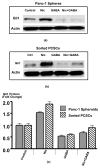
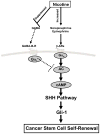
A small subpopulation of pancreatic cancer cells with characteristics of stem cells drive tumour initiation, progression and metastasis. A better understanding of the regulation of cancer stem cells may lead to more effective cancer prevention and therapy. We have shown that the proliferation and migration of pancreatic cancer cell lines is activated by the nicotinic receptor-mediated release of stress neurotransmitters, responses reversed by γ-aminobutyric acid (GABA). However, the observed cancer inhibiting effects of GABA will only succeed clinically if GABA inhibits pancreatic cancer stem cells (PCSCs) in addition to the more differentiated cancer cells that comprise the majority of cancer tissues and cell lines. Using PCSCs isolated from two pancreatic cancer patients by cell sorting and by spheroid formation assay from pancreatic cancer cell line Panc-1, we tested the hypothesis that nicotine induces the self-renewal of PCSCs. Nicotinic acetylcholine receptors (nAChRs) α3, α4, α5 and α7 were expressed and chronic exposure to nicotine increased the protein expression of these receptors. Immunoassays showed that PCSCs produced the stress neurotransmitters epinephrine and norepinephrine and the inhibitory neurotransmitter GABA. Chronic nicotine significantly increased the production of stress neurotransmitters and sonic hedgehog (SHH) while inducing Gli1 protein and decreasing GABA. GABA treatment inhibited the induction of SHH and Gli1. Spheroid formation and 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazoliumbromide assays showed significant nicotine-induced increases in self renewal and cell proliferation, responses blocked by GABA. Our data suggest that nicotine increases the SHH-mediated malignant potential of PCSCs and that GABA prevents these effects.
Keywords: Cancer stem cells; GABA; Nicotinic receptors; Pancreatic cancer; Sonic hedgehog; Stress neurotransmitters.
Copyright © 2015 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

