Non-Coding RNAs in Castration-Resistant Prostate Cancer: Regulation of Androgen Receptor Signaling and Cancer Metabolism
- PMID: 26690121
- PMCID: PMC4691085
- DOI: 10.3390/ijms161226138
Non-Coding RNAs in Castration-Resistant Prostate Cancer: Regulation of Androgen Receptor Signaling and Cancer Metabolism
Abstract
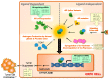
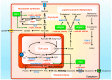
Hormone-refractory prostate cancer frequently relapses from therapy and inevitably progresses to a bone-metastatic status with no cure. Understanding of the molecular mechanisms conferring resistance to androgen deprivation therapy has the potential to lead to the discovery of novel therapeutic targets for type of prostate cancer with poor prognosis. Progression to castration-resistant prostate cancer (CRPC) is characterized by aberrant androgen receptor (AR) expression and persistent AR signaling activity. Alterations in metabolic activity regulated by oncogenic pathways, such as c-Myc, were found to promote prostate cancer growth during the development of CRPC. Non-coding RNAs represent a diverse family of regulatory transcripts that drive tumorigenesis of prostate cancer and various other cancers by their hyperactivity or diminished function. A number of studies have examined differentially expressed non-coding RNAs in each stage of prostate cancer. Herein, we highlight the emerging impacts of microRNAs and long non-coding RNAs linked to reactivation of the AR signaling axis and reprogramming of the cellular metabolism in prostate cancer. The translational implications of non-coding RNA research for developing new biomarkers and therapeutic strategies for CRPC are also discussed.
Keywords: androgen receptor; cancer metabolism; castration-resistant prostate cancer; long non-coding RNAs; micro RNAs; non-coding RNA.
Figures
References
-
- Howlader N., Noone A.M., Krapcho M., Garshell J., Miller D., Altekruse S.F., Kosary C.L., Yu M., Ruhl J., Tatalovich Z., et al. Seer Cancer Statistics review, 1975–2012. National Cancer Institute; Bethesda, MD, USA: 2015.
-
- Dickinson L., Ahmed H.U., Allen C., Barentsz J.O., Carey B., Futterer J.J., Heijmink S.W., Hoskin P.J., Kirkham A., Padhani A.R., et al. Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: Recommendations from a european consensus meeting. Eur. Urol. 2011;59:477–494. doi: 10.1016/j.eururo.2010.12.009. - DOI - PubMed
-
- Rastinehad A.R., Turkbey B., Salami S.S., Yaskiv O., George A.K., Fakhoury M., Beecher K., Vira M.A., Kavoussi L.R., Siegel D.N., et al. Improving detection of clinically significant prostate cancer: Magnetic resonance imaging/transrectal ultrasound fusion guided prostate biopsy. J. Urol. 2014;191:1749–1754. doi: 10.1016/j.juro.2013.12.007. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

