Recent Advances for the Detection of Ochratoxin A
- PMID: 26690216
- PMCID: PMC4690132
- DOI: 10.3390/toxins7124882
Recent Advances for the Detection of Ochratoxin A
Abstract
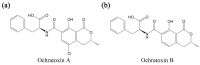
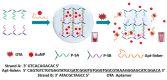
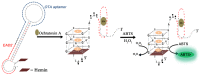
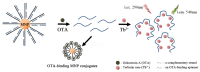
Ochratoxin A (OTA) is one of the mycotoxins secreted by Aspersillus and Penicillium that can easily colonize various grains like coffee, peanut, rice, and maize. Since OTA is a chemically stable compound that can endure the physicochemical conditions of modern food processing, additional research efforts have been devoted to develop sensitive and cost-effective surveillance solutions. Although traditional chromatographic and immunoassays appear to be mature enough to attain sensitivity up to the regulation levels, alternative detection schemes are still being enthusiastically pursued in an attempt to meet the requirements of rapid and cost-effective detections. Herein, this review presents recent progresses in OTA detections with minimal instrumental usage, which have been facilitated by the development of OTA aptamers and by the innovations in functional nanomaterials. In addition to the introduction of aptamer-based OTA detection techniques, OTA-specific detection principles are also presented, which exclusively take advantage of the unique chemical structure and related physicochemical characteristics.
Keywords: amplified detection; aptamers; mycotoxins; ochratoxin A.
Figures
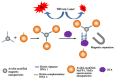
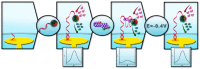

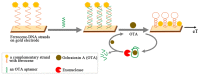
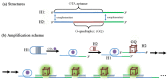
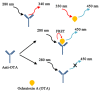
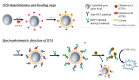
References
-
- Malir F., Ostry V., Novotna E. Toxicity of the mycotoxin ochratoxin A in the light of recent data. Toxin Rev. 2013;32:19–33. doi: 10.3109/15569543.2013.782504. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

