Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants
- PMID: 26690260
- PMCID: PMC9465687
- DOI: 10.1002/14651858.CD010249.pub2
Comparison of animal-derived surfactants for the prevention and treatment of respiratory distress syndrome in preterm infants
Abstract
Background: Animal-derived surfactants have been shown to have several advantages over the first generation synthetic surfactants and are the most commonly used surfactant preparations. The animal-derived surfactants in clinical use are minced or lavaged and modified or purified from bovine or porcine lungs. It is unclear whether significant differences in clinical outcome exist among the available bovine (modified minced or lavage) and porcine (minced or lavage) surfactant extracts.
Objectives: To compare the effect of administration of different animal-derived surfactant extracts on the risk of mortality, chronic lung disease, and other morbidities associated with prematurity in preterm infants at risk for or having respiratory distress syndrome (RDS).
Search methods: We used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7), MEDLINE via PubMed (1966 to July 31, 2015), EMBASE (1980 to July 31, 2015), and CINAHL (1982 to July 31, 2015). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi-randomized trials.
Selection criteria: Randomized or quasi-randomized controlled trials that compared the effect of animal-derived surfactant extract treatment administered to preterm infants at risk for or having RDS to prevent complications of prematurity and mortality.
Data collection and analysis: Data regarding clinical outcomes were excerpted from the reports of the clinical trials by the review authors. Subgroup analyses were performed based on gestational age, surfactant dosing and schedule, treatment severity and treatment strategy. Data analysis was performed in accordance with the standards of the Cochrane Neonatal Review Group.
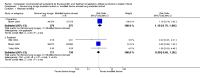
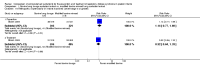
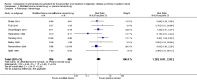
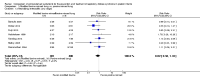
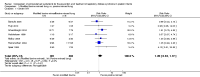
Main results: Sixteen randomized controlled trials were included in the analysis. Bovine lung lavage surfactant extract to modified bovine minced lung surfactant extract: Seven treatment studies and two prevention studies compared bovine lung lavage surfactant extract to modified bovine minced lung surfactant extract. The meta-analysis did not demonstrate any significant differences in death or chronic lung disease in the prevention trials (typical RR 1.02, 95% CI 0.89 to 1.17; typical RD 0.01, 95% CI -0.05 to 0.06; 2 studies and 1123 infants; high quality evidence) or treatment trials (typical RR 0.95, 95% CI 0.86 to 1.06; typical RD -0.02 , 95% CI -0.06 to 0.02; 3 studies and 2009 infants; high quality evidence) Modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract: Nine treatment studies compared modified bovine minced lung surfactant extract to porcine minced lung surfactant extract. Meta-analysis of these trials demonstrate a significant increase in the risk of mortality prior to hospital discharge (typical RR 1.44, 95% CI 1.04 to 2.00; typical RD 0.05, 95% CI 0.01 to 0.10; NNTH 20, 95% CI 10 to 100; 9 studies and 901 infants; moderate quality evidence), death or oxygen requirement at 36 weeks' postmenstrual age (typical RR 1.30, 95% CI 1.04 to 1.64; typical RD 0.11, 95% CI 0.02 to 0.20; NNTH 9, 95% CI 5 to 50; 3 studies and 448 infants; moderate quality evidence), receiving more than one dose of surfactant (typical RR 1.57, 95% CI 1.29 to 1.92; typical RD 0.14, 95% CI 0.08 to 0.20; NNTH 7, 95% CI 5 to 13; 6 studies and 786 infants), and patent ductus arteriosus (PDA) requiring treatment (typical RR 1.86, 95% CI 1.28 to 2.70; typical RD 0.28, 95% CI 0.13 to 0.43; NNTH 4, 95% CI 2 to 8; 3 studies and 137 infants) in infants treated with modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract. In the subgroup analysis based on initial dose of surfactant, improvement in mortality prior to discharge (typical RR 1.62, 95% CI 1.11 to 2.38; typical RD 0.06, 95% CI 0.01 to 0.11; NNTH 16, 95% CI 9 to 100) and risk of death or oxygen requirement at 36 weeks' postmenstrual age (typical RR 1.39, 95% CI 1.08 to 1.79; typical RD 0.13, 95% 0.03 to 0.23; NNTH 7, 95% CI 4 to 33) was limited to higher initial dose of porcine minced lung surfactant (> 100 mg/kg). Other comparisons: No difference in outcome was noted between bovine lung lavage surfactant extract versus porcine minced lung surfactant extract. There were no studies comparing bovine lung lavage surfactant extract versus porcine lung lavage surfactant; or porcine minced lung surfactant extract versus porcine lung lavage surfactant.
Authors' conclusions: Significant differences in clinical outcome were noted in the comparison trials of modified minced lung surfactant extract (beractant) compared with porcine minced lung surfactant extract (poractant alfa) including a significant increase in the risk of mortality prior to discharge, death or oxygen requirement at 36 weeks' postmenstrual age, PDA requiring treatment and "receiving > 1 dose of surfactant" in infants treated with modified bovine minced lung surfactant extract compared with porcine minced lung surfactant extract. The difference in these outcomes was limited to studies using a higher initial dose of porcine minced lung surfactant extract. It is uncertain whether the observed differences are from differences in dose or from source of extraction (porcine vs. bovine) because of the lack of dose-equivalent comparison groups with appropriate sample size. No differences in clinical outcomes were observed in comparative trials between bovine lung lavage surfactant and modified bovine minced lung surfactants.
Conflict of interest statement
Dr Soll has previously acted as a consultant for several of the pharmaceutical companies that manufacture surfactant preparations (Abbott Laboratories, Ross Laboratories, Chiesi Farmaceutici, Dey Laboratories, Burroughs Wellcome). Dr. Soll has not acted as a paid consultant for the past nine years.
Dr Halliday is currently a consultant for Chiesi Farmaceutici, a pharmaceutical company that manufactures a porcine‐derived surfactant preparation; and has been an invited speaker at meetings supported by Abbott Laboratories, Ross Laboratories and Burroughs Wellcome.
Dr Stevens has no known conflicts of interest. This will be further clarified prior to publication of the review.
Dr Singh has no conflict of interest to report.
Dr Suresh has no conflict of interest to report.
Dr. Rojas has no conflict of interest to report.
Figures













































Update of
References
References to studies included in this review
Attar 2004 {published data only}
-
- Attar MA, Becker MA, Dechert RE. Immediate change in lung compliance following natural surfactant administration in premature infants with respiratory distress syndrome. Journal of Perinatology 2004;24(10):626‐30. - PubMed
Baroutis 2003 {published data only}
-
- Baroutis G, Kaleyias J, Liarou T. Comparison of three treatment regimens of the natural surfactant preparations in neonatal respiratory distress syndrome. European Journal of Pediatrics 2003;162(7‐8):476‐80. - PubMed
Bloom 1997 {published data only}
-
- Bloom BT, Kattwinkel J, Hall RT, Delmore PM, Egan EA, Trout JR, et al. Comparison of Infasurf (calf lung surfactant extract) to Survanta (Beractant) in the treatment and prevention of respiratory distress syndrome. Pediatrics 1997;100(1):31‐8. - PubMed
Bloom 2005 {published data only}
-
- Bloom BT, Clark RH for Infasurf Survanta Clinical Trial group. Comparision of Infasurf (calfactant) and Survanta (beractant) in the prevention of respiratory distress syndrome. Pediatrics 2005;116(2):392‐9. - PubMed
Didzar 2012 {published data only}
-
- Dizdar EA, Sari FN, Aydemir C, Oguz SS, Erdeve O, Uras N, et al. A randomized, controlled trial of poractant alfa versus beractant in the treatment of preterm infants with respiratory distress syndrome. American Journal of Perinatology 2012;29(2):95‐100. - PubMed
Fujii 2010 {published data only}
-
- Fujii AM, Patel SM, Allen R. Poractant alfa and beractant treatment of very premature infants with respiratory distress syndrome. Journal of Perinatology 2010;30(10):665‐70. - PubMed
Gharehbaghi 2010 {published data only}
-
- Gharehbaghi MM, Sakha SH, Ghojazadeh M, Firoozi F. Complications among premature neonates treated with beractant and poractant alfa. Indian Journal of Pediatrics 2010;77(7):751‐4. - PubMed
Halahakoon 1999 {published data only}
-
- Halahakoon WL. A study of cerebral function following surfactant treatment for respiratory distress syndrome (Doctoral dissertation). Queen's University of Belfast (UK) 1999.
Hammoud 2004 {published data only}
-
- Hammoud M, Al‐Kazmi N, Alshemmiri M, Thalib L, Ranjani VT, Devarajan LV, et al. Randomized clinical trial comparing two natural surfactant preparations to treat respiratory distress syndrome. Journal of Maternal Fetal and Neonatal Medicine 2004;15(3):167‐75. - PubMed
Karadag 2014 {published data only}
-
- Karadag N, Dilli D, Zenciroglu A, Aydin B, Beken S, Okumus N. Perfusion index variability in preterm infants treated with two different natural surfactants for respiratory distress syndrome. American Journal of Perinatology 2014;31(11):1015‐22. [PUBMED: 24566756] - PubMed
Lam 2005 {published data only}
-
- Lam BC, Ng YK, Wong KY. Randomized trial comparing two natural surfactants (Survanta vs bLES) for treatment of neonatal respiratory distress syndrome. Pediatric Pulmonology 2005;39(1):64‐9. - PubMed
Malloy 2005 {published data only}
-
- Malloy CA, Nicoski P, Muraskas JK. A randomized trial comparing beractant and poractant treatment in neonatal respiratory distress syndrome. Acta Paediatrica 2005;94(6):779‐84. - PubMed
Ramanathan 2004 {published data only}
-
- Ramanathan R, Rasmussen MR, Gerstmann DR, Finer N, Sekar K, North American Study Group. A randomized, multicenter masked comparison trial of poractant alfa (Curosurf) versus beractant (Survanta) in the treatment of respiratory distress syndrome in preterm infants. American Journal of Perinatology 2004;21(3):109‐19. - PubMed
Sanchez‐Mendiola 2005 {published data only}
-
- Sánchez‐Mendiola M, Martínez‐Natera OC, Herrera‐Maldonado N, Ortega‐Arroyo J. Treatment of hyaline membrane disease in the preterm newborn with exogenous lung surfactant: a controlled study [Estudio controlado del tratamiento de la enfermedad de membrana hialina del recien nacido pretermino con surfactante pulmonar exogeno (porcino vs bovino)]. Gaceta medica Mexico 2005;141(4):267‐71. - PubMed
Speer 1995 {published data only}
Yalaz 2004 {published data only}
-
- Yalaz M, Aslanoglu S, Akisu M, Atik T, Ergun O, Kultursay N. A comparison of efficacy between two natural exogenous surfactant preparations in premature infants with respiratory distress syndrome. Klinische Padiatrie 2004;216(4):230‐5. - PubMed
References to studies excluded from this review
Bozdağ 2015 {published data only}
-
- Bozdağ S, Dilli D, Gökmen T, Dilmen U. Comparison of two natural surfactants for pulmonary hemorrhage in very low‐birth‐weight infants: a randomized controlled trial. American Journal of Perinatology 2015;32(3):211‐8. - PubMed
Choi 2005 {published data only}
Proquitté 2007 {published data only}
-
- Proquitté H, Dushe T, Hammer H, Rüdiger M, Schmalisch, G, Wauer RR, et al. Observational study to compare the clinical efficacy of the natural surfactants Alveofact and Curosurf in the treatment of respiratory distress syndrome in premature infants. Respiratory Medicine 2007;101(1):169‐76. - PubMed
Rebello 2009 {unpublished data only}
-
- Rebello CM, Precioso AR, Mascaretti RS, Grupo Colaborativo do Estudo Brasileiro Multicêntrico de Surfactante. A multicenter, randomized, double‐blind trial of a new porcine surfactant in premature infants with respiratory distress syndrome. Einstein (Sao Paulo) 2014;12(4):397‐404. [DOI: 10.1590/S1679-45082014AO3095] - DOI - PMC - PubMed
References to studies awaiting assessment
Eras 2014 {published data only}
-
- Eras Z, Dizdar EA, Kanmaz G, Guzoglu N, Aksoy HT, Altunkaya GB, et al. Neurodevelopmental outcomes of very low birth weight preterm infants treated with poractant alfa versus beractant for respiratory distress syndrome. American Journal of Perinatology 2014;31(6):463‐8. [DOI: 10.1055/s-0033-1351659] - DOI - PubMed
Gharehbaghi 2014 {published data only}
-
- Gharehbaghi MM, Yasrebi S. Comparing the efficacy of two natural surfactants, Curosurf and Alveofact, in treatment of respiratory distress syndrome in preterm infants. International Journal of Women’s Health and Reproduction Sciences 2014;2(4):245‐8.
Mercado 2010 {published data only}
-
- Mercado VV, Cristea I, Ali N, Pham CC, Buescher E, Yang J, et al. Does surfactant type cause a differential proinflammatory response in preterm infant with respiratory distress syndrome?. Advances in Therapy 2010;27(7):476‐82. - PubMed
Saeidi 2013 {published data only}
-
- Saeidi R, Hamedi A, Javadi A, Robatsangi MG, Dinparvar SK. Comparison of side effects of survanta and curosurf in decreasing mortality due to respiratory distress syndrome (RDS) in premature infants admitted in NICU of Ghaem Hospital on 2006‐2008. Iranian Journal of Neonatology 2013;4(3):7‐12. [AN:2013708341]
Terek 2015 {published data only}
-
- Terek D, Gonulal D, Koroglu OA, Yalaz M, Akisu M, Kultursay N. Effects of two different exogenous surfactant preparations on serial peripheral perfusion index and tissue carbon monoxide measurements in preterm infants with severe respiratory distress syndrome. Pediatric Neurology 2015;56(4):248‐55. - PubMed
Additional references
Avery 1959
-
- Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. American Journal of Diseases in Children 1959;97(5 Pt 1):517‐23. - PubMed
Bell 1978
Chu 1967
-
- Chu J, Clements JA, Cotton EK, Klaus MH, Sweet AY, Tooley WH. Neonatal pulmonary ischemia. I. Clinical and physiological studies. Pediatrics 1967;40(4):709‐82. - PubMed
Dargaville 2011
-
- Dargaville PA, Aiyappan A, Cornelius A, Williams C, Antonio G De Paoli. Preliminary evaluation of a new technique of minimally invasive surfactant therapy. Archive of Disease in Childhood ‐ Fetal and Neonatal Edition 2011;96(4):F243–8. - PubMed
Engle 2008
-
- Engle WA, American Academy of Pediatrics Committee on Fetus and Newborn. Surfactant‐replacement therapy for respiratory distress in the preterm and term neonate. Pediatrics 2008;121(2):419‐32. - PubMed
Enhorning 1972
-
- Enhorning G, Robertson B. Lung expansion in the premature rabbit fetus after tracheal deposition of surfactant. Pediatrics 1972;50(1):58‐66. - PubMed
Fujiwara 1980
-
- Fujiwara T, Maeta H, Chida S, Morita T, Watabe YJ, Abe T. Artificial surfactant therapy in hyaline membrane disease. Lancet 1980;I(8159):55‐9. - PubMed
GradePro 2008 [Computer program]
-
- Jan Brozek, Andrew Oxman, Holger Schünemann. GradePro [Version 3.2 for Windows]. 2008.
Guyatt 2011a
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of clinical epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] - PubMed
Guyatt 2011b
-
- Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso‐Coello P, et al. GRADE guidelines: 4. Rating the quality of evidence‐‐study limitations (risk of bias). Journal of Clinical Epidemiology 2011;64(4):407‐15. [PUBMED: 21247734] - PubMed
Guyatt 2011c
-
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of clinical epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] - PubMed
Guyatt 2011d
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 7. Rating the quality of evidence‐‐inconsistency. Journal of clinical epidemiology 2011;64(12):1294‐302. [PUBMED: 21803546] - PubMed
Guyatt 2011e
-
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence‐‐indirectness. Journal of clinical epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] - PubMed
Halliday 2008
Hawgood 1985
-
- Hawgood S, Benson BJ, Hamilton Jr RL. Effects of surfactant‐associated proteins and calcium ions on the structure and surface activity of lung surfactant lipids. Biochemistry 1985;24(1):184‐90. - PubMed
Higgins 2011
-
- Higgins A, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICCROP 2005
-
- International Committee for the Classification of Retinopathy of Prematurity. The International Classification of Retinopathy of Prematurity revisited. Archives of Ophthalmology 2005;123(7):991‐9. - PubMed
Jobe 1993
-
- Jobe AH. Pulmonary surfactant therapy. New England Journal of Medicine 1993;328(1):861‐8. - PubMed
Kribs 2007
-
- Kribs A, Pillekamp F, Hunseler C. Early administration of surfactant in spontaneous breathing with nCPAP: feasibility and outcome in extremely premature infants (postmenstrual age < 27 weeks). Pediatric Anesthesia 2007;17(4):364‐9. - PubMed
Moya 2009
Papile 1978
-
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. - PubMed
Pfister 2007
Pfister 2009
Ramanathan 2009
-
- Ramanathan R. Animal derived surfactants: where are we? The evidence from randomized controlled trials. Journal of Perinatology 2009;29(Suppl 2):s38‐43. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robillard 1964
Rojas‐Reyes 2012
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook. Updated October 2013.
Seger 2009
Singh 2011
-
- Singh N, Hawley KL, Viswanathan K. Efficacy of porcine versus bovine surfactants for preterm newborns with respiratory distress syndrome: systematic review and meta‐analysis. Pediatrics 2011;128(6):e1588‐95. - PubMed
Soll 1992
-
- Soll RF, McQueen MC. Respiratory Distress Syndrome. Sinclair J, Bracken M: Effective Care of the Newborn Infant. New York: Oxford University Press, 1992:325‐58. [ISBN 0‐19‐261737‐0]
Soll 1997
Soll 1998
Soll 2001
Soll 2010
Sweet 2013
-
- Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of neonatal respiratory distress syndrome in preterm infants‐‐2013 update. Neonatology 2013;103(4):353‐68. - PubMed
Whitsett 1995
-
- Whitsett JA, Nogee LM, Weaver TE, Horowitz AD. Human surfactant protein B: structure, function, regulation, and genetic disease. Physiological Reviews 1995;75(4):749‐57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

