HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials
- PMID: 26690310
- PMCID: PMC4785607
- DOI: 10.1002/path.4679
HER2 overexpression and amplification as a potential therapeutic target in colorectal cancer: analysis of 3256 patients enrolled in the QUASAR, FOCUS and PICCOLO colorectal cancer trials
Abstract
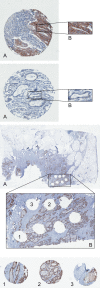
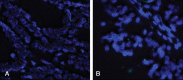
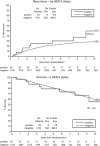
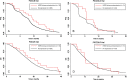
HER2 overexpression/amplification is linked to trastuzumab response in breast/gastric cancers. One suggested anti-EGFR resistance mechanism in colorectal cancer (CRC) is aberrant MEK-AKT pathway activation through HER2 up-regulation. We assessed HER2-amplification/overexpression in stage II-III and IV CRC patients, assessing relationships to KRAS/BRAF and outcome. Pathological material was obtained from 1914 patients in the QUASAR stage II-III trial and 1342 patients in stage IV trials (FOCUS and PICCOLO). Tissue microarrays were created for HER2 immunohistochemistry. HER2-amplification was assessed using FISH and copy number variation. KRAS/BRAF mutation status was assessed by pyrosequencing. Progression-free survival (PFS) and overall survival (OS) data were obtained for FOCUS/PICCOLO and recurrence and mortality for QUASAR; 29/1342 (2.2%) stage IV and 25/1914 (1.3%) stage II-III tumours showed HER2 protein overexpression. Of the HER2-overexpressing cases, 27/28 (96.4%) stage IV tumours and 20/24 (83.3%) stage II-III tumours demonstrated HER2 amplification by FISH; 41/47 (87.2%) also showed copy number gains. HER2-overexpression was associated with KRAS/BRAF wild-type (WT) status at all stages: in 5.2% WT versus 1.0% mutated tumours (p < 0.0001) in stage IV and 2.1% versus 0.2% in stage II-III tumours (p = 0.01), respectively. HER2 was not associated with OS or PFS. At stage II-III, there was no significant correlation between HER2 overexpression and 5FU/FA response. A higher proportion of HER2-overexpressing cases experienced recurrence, but the difference was not significant. HER2-amplification/overexpression is identifiable by immunohistochemistry, occurring infrequently in stage II-III CRC, rising in stage IV and further in KRAS/BRAF WT tumours. The value of HER2-targeted therapy in patients with HER2-amplified CRC must be tested in a clinical trial. © 2015 The Authors. Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Keywords: HER2; amplification; colorectal cancer; overexpression, copy number variation.
© 2015 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures
References
-
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61: 69–90. - PubMed
-
- Wells A. EGF receptor. Int J Biochem Cell Biol 1999; 31: 637–643. - PubMed
-
- Press MF, Bernstein L, Thomas PA, et al. HER‐2/neu gene amplification characterized by fluorescence in situ hybridization: poor prognosis in node‐negative breast carcinomas. J Clin Oncol 1997; 15: 2894–2904. - PubMed
-
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER‐2/neu oncogene. Science 1987; 235: 177–182. - PubMed
-
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER‐2/neu proto‐oncogene in human breast and ovarian cancer. Science 1989; 244: 707–712. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

