Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae)
- PMID: 26690400
- PMCID: PMC6332510
- DOI: 10.3390/molecules201219829
Leishmanicidal Activity of (+)-Phyllanthidine and the Phytochemical Profile of Margaritaria nobilis (Phyllanthaceae)
Abstract
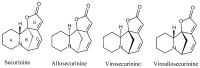
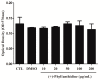
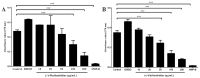
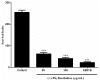
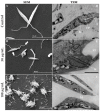
The effects of the Securinega alkaloid (+)-phyllanthidine on Leishmania (L.) amazonensis and the first chemical investigation of Margaritaria nobilis L.f. (Phyllanthaceae) are described. Treating the parasites with this alkaloid caused a dose-dependent reduction in promastigote growth of 67.68% (IC50 82.37 μg/mL or 353 µM) and in amastigote growth of 83.96% (IC50 49.11 μg/mL or 210 µM), together with ultrastructural alterations in the promastigotes. No cytotoxic effect was detected in mammalian cells (CC50 1727.48 µg/mL or CC50 5268 µM). Classical chromatographic techniques and spectral methods led to the isolation and identification of betulinic acid, kaempferol, corilagin, gallic acid and its methyl ester, besides (+)-phyllanthidine from M. nobilis leaves and stems. Margaritaria nobilis is another source of the small group of Securinega alkaloids, together with other Phyllanthaceae (Euphorbiaceae s.l.) species. The low toxicity to macrophages and the effects against promastigotes and amastigotes are suggestive that (+)-phyllanthidine could be a promising antileishmanial agent for treating cutaneous leishmaniasis.
Keywords: Leishmania (L.) amazonensis; alkaloid (+)-phyllanthidine; leishmanicidal activity.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Antileishmanial activity and trypanothione reductase effects of terpenes from the Amazonian species Croton cajucara Benth (Euphorbiaceae).Phytomedicine. 2015 Nov 15;22(12):1133-7. doi: 10.1016/j.phymed.2015.08.012. Epub 2015 Sep 18. Phytomedicine. 2015. PMID: 26547537
-
Leishmanicidal activity of synthetic chalcones in Leishmania (Viannia) braziliensis.Exp Parasitol. 2014 Jan;136:27-34. doi: 10.1016/j.exppara.2013.11.003. Epub 2013 Nov 21. Exp Parasitol. 2014. PMID: 24269198
-
Chemical characterisation of the constituents of Eugenia protenta McVaugh and leishmanicidal activity of dimethylxanthoxylin.Nat Prod Res. 2019 Mar;33(6):879-883. doi: 10.1080/14786419.2017.1410804. Epub 2017 Dec 6. Nat Prod Res. 2019. PMID: 29212369
-
Leishmanicidal effect of Spiranthera odoratíssima (Rutaceae) and its isolated alkaloid skimmianine occurs by a nitric oxide dependent mechanism.Parasitology. 2011 Sep;138(10):1224-33. doi: 10.1017/S0031182011001168. Epub 2011 Aug 3. Parasitology. 2011. PMID: 21810308
-
Evaluation of the antileishmanial potency, toxicity and phytochemical constituents of methanol bark extract of Sterculia villosa.Pharm Biol. 2017 Dec;55(1):998-1009. doi: 10.1080/13880209.2017.1285946. Pharm Biol. 2017. PMID: 28173714 Free PMC article.
Cited by
-
Margaritaria nobilis L.F. (Phyllanthaceae): Ethnopharmacology and Application of Computational Tools in the Annotation of Bioactive Molecules.Metabolites. 2022 Jul 25;12(8):681. doi: 10.3390/metabo12080681. Metabolites. 2022. PMID: 35893248 Free PMC article.
-
Total Synthesis of (±)-Phyllantidine: Development and Mechanistic Evaluation of a Ring Expansion for Installation of Embedded Nitrogen-Oxygen Bonds.Angew Chem Int Ed Engl. 2020 Jun 8;59(24):9757-9766. doi: 10.1002/anie.202003829. Epub 2020 May 8. Angew Chem Int Ed Engl. 2020. PMID: 32271982 Free PMC article.
-
Polymeric nanoparticles containing kojic acid induce structural alterations and apoptosis-like death in Leishmania (Leishmania) amazonensis.Front Pharmacol. 2024 Jan 23;15:1331240. doi: 10.3389/fphar.2024.1331240. eCollection 2024. Front Pharmacol. 2024. PMID: 38323082 Free PMC article.
-
Asymmetric synthesis of chiral 1,2-oxazinane and hexahydropyridazin spirocyclic scaffolds by organocatalytic [4 + 2] cycloaddition.RSC Adv. 2022 May 24;12(25):15713-15717. doi: 10.1039/d2ra02759c. eCollection 2022 May 23. RSC Adv. 2022. PMID: 35685709 Free PMC article.
-
In Vitro Antileishmanial Activity of Sterols from Trametes versicolor (Bres. Rivarden).Molecules. 2016 Aug 10;21(8):1045. doi: 10.3390/molecules21081045. Molecules. 2016. PMID: 27517895 Free PMC article.
References
-
- WHO World Health Organization (2015). Control of the Leishmaniases. Fact Sheets, 375. [(accessed on 22 February 2015)]. Available online: http://www.who.int/mediacentre/factsheets/fs375/en/
-
- WHO Expert Committee (2010). Control of the Leishmaniases; Geneva. [(accessed on 13 March 2010)]. pp. 22–26. WHO Technical Report Series; No. 949. Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases. Available online: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3307017&tool=p....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

