Fingolimod (FTY720-P) Does Not Stabilize the Blood-Brain Barrier under Inflammatory Conditions in an in Vitro Model
- PMID: 26690412
- PMCID: PMC4691120
- DOI: 10.3390/ijms161226177
Fingolimod (FTY720-P) Does Not Stabilize the Blood-Brain Barrier under Inflammatory Conditions in an in Vitro Model
Abstract
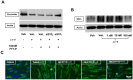
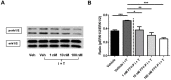
Breakdown of the blood-brain barrier (BBB) is an early hallmark of multiple sclerosis (MS), a progressive inflammatory disease of the central nervous system. Cell adhesion in the BBB is modulated by sphingosine-1-phosphate (S1P), a signaling protein, via S1P receptors (S1P₁). Fingolimod phosphate (FTY720-P) a functional S1P₁ antagonist has been shown to improve the relapse rate in relapsing-remitting MS by preventing the egress of lymphocytes from lymph nodes. However, its role in modulating BBB permeability-in particular, on the tight junction proteins occludin, claudin 5 and ZO-1-has not been well elucidated to date. In the present study, FTY720-P did not change the transendothelial electrical resistance in a rat brain microvascular endothelial cell (RBMEC) culture exposed to inflammatory conditions and thus did not decrease endothelial barrier permeability. In contrast, occludin was reduced in RBMEC culture after adding FTY720-P. Additionally, FTY720-P did not alter the amount of endothelial matrix metalloproteinase (MMP)-9 and MMP-2 in RBMEC cultures. Taken together, our observations support the assumption that S1P₁ plays a dual role in vascular permeability, depending on its ligand. Thus, S1P₁ provides a mechanistic basis for FTY720-P-associated disruption of endothelial barriers-such as the blood-retinal barrier-which might result in macular edema.
Keywords: FTY720-P; blood-brain barrier; inflammation; rat brain microvascular endothelial cell culture; tight junctions.
Figures








Similar articles
-
Fingolimod prevents blood-brain barrier disruption induced by the sera from patients with multiple sclerosis.PLoS One. 2015 Mar 16;10(3):e0121488. doi: 10.1371/journal.pone.0121488. eCollection 2015. PLoS One. 2015. PMID: 25774903 Free PMC article.
-
Sphingosine 1-phosphate signaling at the blood-brain barrier.Trends Mol Med. 2015 Jun;21(6):354-63. doi: 10.1016/j.molmed.2015.03.006. Epub 2015 May 1. Trends Mol Med. 2015. PMID: 25939882
-
FTY720 rescue therapy in the dark agouti rat model of experimental autoimmune encephalomyelitis: expression of central nervous system genes and reversal of blood-brain-barrier damage.Brain Pathol. 2009 Apr;19(2):254-66. doi: 10.1111/j.1750-3639.2008.00182.x. Epub 2008 Jun 4. Brain Pathol. 2009. PMID: 18540945 Free PMC article.
-
Central nervous system-directed effects of FTY720 (fingolimod).J Neurol Sci. 2008 Nov 15;274(1-2):13-7. doi: 10.1016/j.jns.2008.06.031. Epub 2008 Aug 3. J Neurol Sci. 2008. PMID: 18678377 Review.
-
[Fingolimod therapy in multiple sclerosis--the issue of the pathomechanism].Ideggyogy Sz. 2012 Mar 30;65(3-4):83-100. Ideggyogy Sz. 2012. PMID: 23136726 Review. Hungarian.
Cited by
-
FTY720 Protects Against Ischemia-Reperfusion Injury by Preventing the Redistribution of Tight Junction Proteins and Decreases Inflammation in the Subacute Phase in an Experimental Stroke Model.Transl Stroke Res. 2020 Oct;11(5):1103-1116. doi: 10.1007/s12975-020-00789-x. Epub 2020 Feb 27. Transl Stroke Res. 2020. PMID: 32103462 Free PMC article.
-
Sphingolipid Metabolism in Glioblastoma and Metastatic Brain Tumors: A Review of Sphingomyelinases and Sphingosine-1-Phosphate.Biomolecules. 2020 Sep 23;10(10):1357. doi: 10.3390/biom10101357. Biomolecules. 2020. PMID: 32977496 Free PMC article. Review.
-
Electrical Stimulation of the Mesencephalic Locomotor Region Has No Impact on Blood-Brain Barrier Alterations after Cerebral Photothrombosis in Rats.Int J Mol Sci. 2019 Aug 19;20(16):4036. doi: 10.3390/ijms20164036. Int J Mol Sci. 2019. PMID: 31430854 Free PMC article.
-
Effects of Fullerenols on Mouse Brain Microvascular Endothelial Cells.Int J Mol Sci. 2017 Aug 17;18(8):1783. doi: 10.3390/ijms18081783. Int J Mol Sci. 2017. PMID: 28817067 Free PMC article.
-
Blood-brain barrier dysfunction in hepatic encephalopathy: pathophysiology, diagnostic assessment and therapeutic perspectives.Metab Brain Dis. 2025 Jun 13;40(5):223. doi: 10.1007/s11011-025-01645-3. Metab Brain Dis. 2025. PMID: 40512384 Review.
References
-
- Alt C., Duvefelt K., Franzén B., Yang Y., Engelhardt B. Gene and protein expression profiling of the microvascular compartment in experimental autoimmune encephalomyelitis in C57Bl/6 and SJL mice. Brain Pathol. Zurich Switz. 2005;15:1–16. doi: 10.1111/j.1750-3639.2005.tb00094.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

