Engaging stakeholders to design a comparative effectiveness trial in children with uncontrolled asthma
- PMID: 26690579
- PMCID: PMC4900169
- DOI: 10.2217/cer.15.52
Engaging stakeholders to design a comparative effectiveness trial in children with uncontrolled asthma
Erratum in
-
Corrigendum.J Comp Eff Res. 2016 Mar;5(2):228. doi: 10.2217/cer.15.52101. Epub 2016 Feb 8. J Comp Eff Res. 2016. PMID: 26852882 Free PMC article. No abstract available.
Abstract
Aim: To present the methods and outcomes of stakeholder engagement in the development of interventions for children presenting to the emergency department (ED) for uncontrolled asthma.
Methods: We engaged stakeholders (caregivers, physicians, nurses, administrators) from six EDs in a three-phase process to: define design requirements; prototype and refine; and evaluate.
Results: Interviews among 28 stakeholders yielded themes regarding in-home asthma management practices and ED discharge experiences. Quantitative and qualitative evaluation showed strong preference for the new discharge tool over current tools.
Conclusion: Engaging end-users in contextual inquiry resulted in CAPE (CHICAGO Action Plan after ED discharge), a new stakeholder-balanced discharge tool, which is being tested in a multicenter comparative effectiveness trial.
Keywords: asthma; health communication; patient discharge; pediatrics; stakeholder engagement; written action plan.
Conflict of interest statement
The study was sponsored by the Patient-Centered Outcomes Research Institute (contract #AS-1307-05420). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Figures




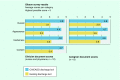
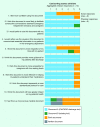
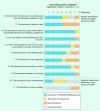
References
-
- Comparative Effectiveness Research: National Institutes of Health. PCAST Session on Health Reform and CER. 2009. www.whitehouse.gov/files/documents/ostp/PCAST/Nabel%20Presentation.pdf
-
- National Institutes of Health/National Heart, Lung, and Blood Institute. Expert Panel Report 3: guidelines on asthma. www.nhlbi.nih.gov/health-pro/guidelines/current/asthma-guidelines/full-r...
-
- Global Initiative for Asthma (GINA) www.ginasthma.org/
-
- Gupta S, Wan FT, Ducharme FM, Chignell MH, Lougheed MD, Straus SE. Asthma action plans are highly variable and do not conform to best visual design practices. Ann. Allergy Asthma Immunol. 2012;108(4):260–265. - PubMed
-
- Ring N, Jepson R, Hoskins G, et al. Understanding what helps or hinders asthma action plan use: a systematic review and synthesis of the qualitative literature. Patient Educ. Couns. 2011;85(2):e131–e143. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
