Up-regulation of Orai1 expression and store operated Ca(2+) entry following activation of membrane androgen receptors in MCF-7 breast tumor cells
- PMID: 26690689
- PMCID: PMC4687293
- DOI: 10.1186/s12885-015-2014-2
Up-regulation of Orai1 expression and store operated Ca(2+) entry following activation of membrane androgen receptors in MCF-7 breast tumor cells
Abstract
Background: Membrane androgen receptors (mAR) are functionally expressed in a variety of tumor-cells including the breast tumor-cell line MCF-7. They are specifically activated by testosterone albumin conjugates (TAC). The mAR sensitive signaling includes activation of Ras-related C3 botulinum toxin substrate 1 (Rac1) and reorganization of the actin filament network. Signaling of tumor-cells may further involve up-regulation of pore forming Ca(2+) channel protein Orai1, which accomplishes store operated Ca(2+) entry (SOCE). This study explored the regulation of Orai1 abundance and SOCE by mAR.
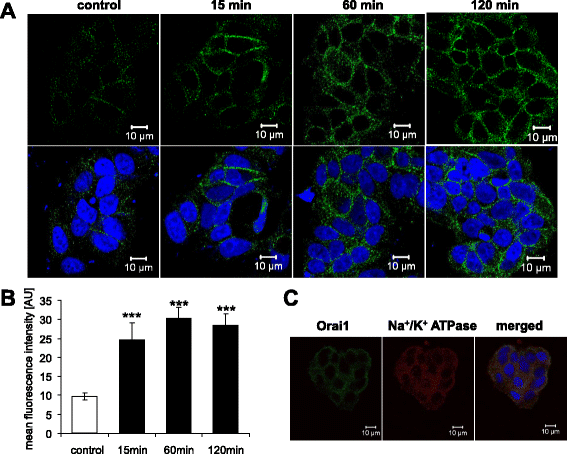
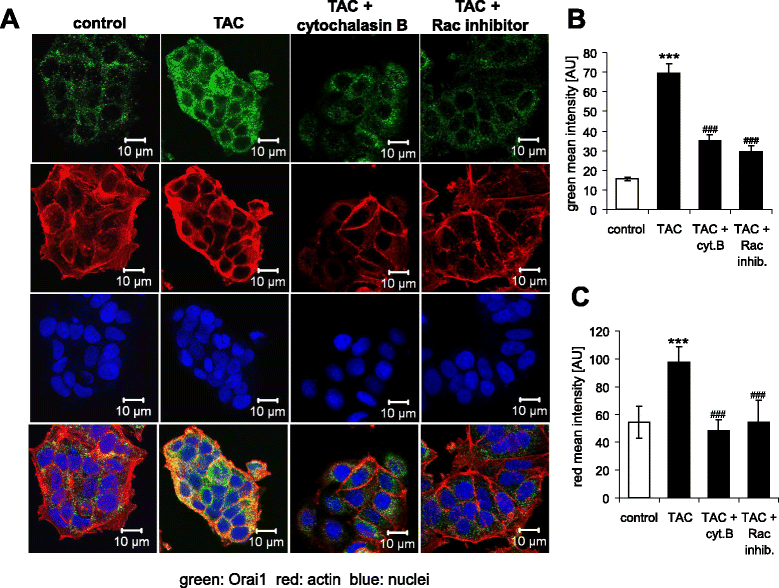
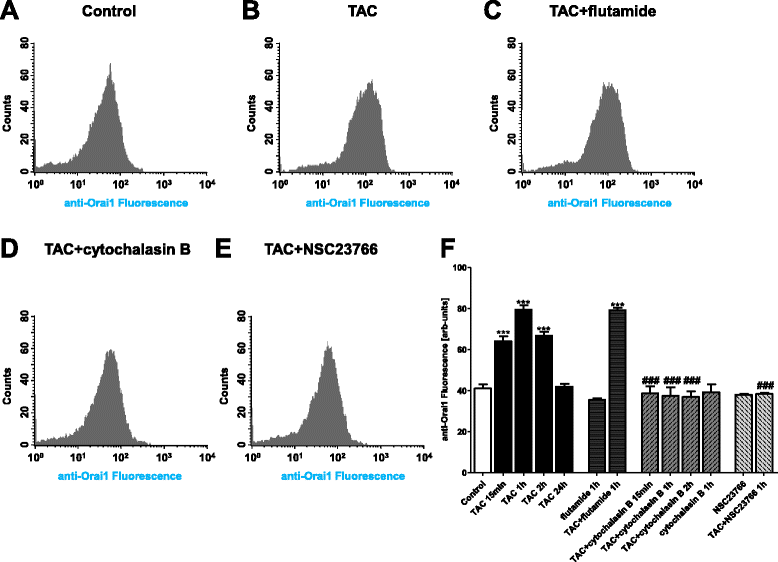
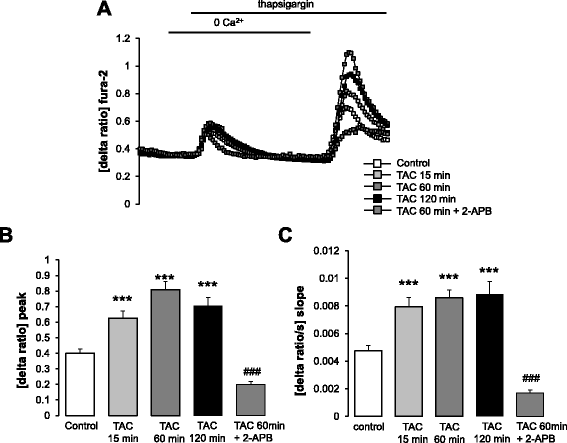
Methods: Actin filaments were visualized utilizing confocal microscopy, Rac1 activity using GST-GBD assay, Orai1 transcript levels by RT-PCR and total protein abundance by western blotting, Orai1 abundance at the cell surface by confocal microscopy and FACS-analysis, cytosolic Ca(2+) activity ([Ca(2+)]i) utilizing Fura-2-fluorescence, and SOCE from increase of [Ca(2+)]i following readdition of Ca(2+) after store depletion with thapsigargin (1 μM).
Results: TAC treatment of MCF-7 cells was followed by Rac1 activation, actin polymerization, transient increase of Orai1transcript levels and protein abundance, and transient increase of SOCE. The transient increase of Orai1 protein abundance was abrogated by Rac1 inhibitor NSC23766 (50 μM) and by prevention of actin reorganization with cytochalasin B (1 μM).
Conclusions: mAR sensitive Rac1 activation and actin reorganization contribute to the regulation of Orai1 protein abundance and SOCE.
Figures




References
-
- Kampa M, Papakonstanti EA, Hatzoglou A, Stathopoulos EN, Stournaras C, Castanas E. The human prostate cancer cell line LNCaP bears functional membrane testosterone receptors that increase PSA secretion and modify actin cytoskeleton. FASEB J. 2002;16:1429–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

