Oral versus intravenous antibiotic treatment for bone and joint infections (OVIVA): study protocol for a randomised controlled trial
- PMID: 26690812
- PMCID: PMC4687165
- DOI: 10.1186/s13063-015-1098-y
Oral versus intravenous antibiotic treatment for bone and joint infections (OVIVA): study protocol for a randomised controlled trial
Abstract
Background: Bone and joint infection in adults arises most commonly as a complication of joint replacement surgery, fracture fixation and diabetic foot infection. The associated morbidity can be devastating to patients and costs the National Health Service an estimated £20,000 to £40,000 per patient. Current standard of care in most UK centres includes a prolonged course (4-6 weeks) of intravenous antibiotics supported, if available, by an outpatient parenteral antibiotic therapy service. Intravenous therapy carries with it substantial risks and inconvenience to patients, and the antibiotic-related costs are approximately ten times that of oral therapy. Despite this, there is no evidence to suggest that oral therapy results in inferior outcomes. We hypothesise that, by selecting oral agents with high bioavailability, good tissue penetration and activity against the known or likely pathogens, key outcomes in patients managed primarily with oral therapy are non-inferior to those in patients treated by intravenous therapy.
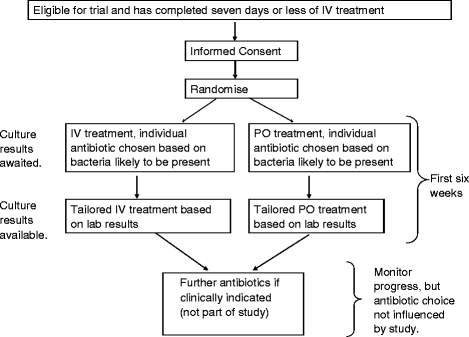
Methods: The OVIVA trial is a parallel group, randomised (1:1), un-blinded, non-inferiority trial conducted in thirty hospitals across the UK. Eligible participants are adults (>18 years) with a clinical syndrome consistent with a bone, joint or metalware-associated infection who have received ≤7 days of intravenous antibiotic therapy from the date of definitive surgery (or the start of planned curative therapy in patients treated without surgical intervention). Participants are randomised to receive either oral or intravenous antibiotics, selected by a specialist infection physician, for the first 6 weeks of therapy. The primary outcome measure is definite treatment failure within one year of randomisation, as assessed by a blinded endpoint committee, according to pre-defined microbiological, histological and clinical criteria. Enrolling 1,050 subjects will provide 90 % power to demonstrate non-inferiority, defined as less than 7.5 % absolute increase in treatment failure rate in patients randomised to oral therapy as compared to intravenous therapy (one-sided alpha of 0.05).
Discussion: If our results demonstrate non-inferiority of orally administered antibiotic therapy, this trial is likely to facilitate a dramatically improved patient experience and alleviate a substantial financial burden on healthcare services.
Trial registration: ISRCTN91566927 - 14/02/2013.
Figures
Similar articles
-
Oral versus intravenous antibiotics for bone and joint infections: the OVIVA non-inferiority RCT.Health Technol Assess. 2019 Aug;23(38):1-92. doi: 10.3310/hta23380. Health Technol Assess. 2019. PMID: 31373271 Free PMC article. Clinical Trial.
-
Oral versus Intravenous Antibiotics for Bone and Joint Infection.N Engl J Med. 2019 Jan 31;380(5):425-436. doi: 10.1056/NEJMoa1710926. N Engl J Med. 2019. PMID: 30699315 Free PMC article. Clinical Trial.
-
Oral versus intravenous empirical antibiotics in children and adolescents with uncomplicated bone and joint infections: a nationwide, randomised, controlled, non-inferiority trial in Denmark.Lancet Child Adolesc Health. 2024 Sep;8(9):625-635. doi: 10.1016/S2352-4642(24)00133-0. Epub 2024 Jul 15. Lancet Child Adolesc Health. 2024. PMID: 39025092 Clinical Trial.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
Cited by
-
The effectiveness of systemic antibiotics for osteomyelitis of the foot in adults with diabetes mellitus: a systematic review protocol.J Foot Ankle Res. 2022 Jun 17;15(1):48. doi: 10.1186/s13047-022-00554-3. J Foot Ankle Res. 2022. PMID: 35710432 Free PMC article.
-
Staphylococcal Osteomyelitis: Disease Progression, Treatment Challenges, and Future Directions.Clin Microbiol Rev. 2018 Feb 14;31(2):e00084-17. doi: 10.1128/CMR.00084-17. Print 2018 Apr. Clin Microbiol Rev. 2018. PMID: 29444953 Free PMC article. Review.
-
Safety and Efficacy of Prolonged Use of Dalbavancin in Bone and Joint Infections.Antimicrob Agents Chemother. 2019 Apr 25;63(5):e02280-18. doi: 10.1128/AAC.02280-18. Print 2019 May. Antimicrob Agents Chemother. 2019. PMID: 30858217 Free PMC article.
-
Antimicrobial activity of mesenchymal stem cells against Staphylococcus aureus.Stem Cell Res Ther. 2020 Jul 17;11(1):293. doi: 10.1186/s13287-020-01807-3. Stem Cell Res Ther. 2020. PMID: 32680544 Free PMC article.
-
[Management of fracture-related infections].Unfallchirurg. 2022 Jan;125(1):41-49. doi: 10.1007/s00113-021-01116-1. Epub 2021 Dec 21. Unfallchirurg. 2022. PMID: 34932139 Review. German.
References
-
- National Joint Registry. NJR 10th Annual Report 2013; ISSN 2054-183X. http://www.njrcentre.org.uk/njrcentre/Portals/0/Documents/England/Report.... Accessed 10 March 2015.
-
- Public Health England. Surveillance of surgical site infections in NHS hospitals in England, 2013/2014. London: Public Health England. https://www.gov.uk/government/uploads/system/uploads/attachment_data/fil.... Accessed 10 March 2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical