A small multigene hydroxyproline-O-galactosyltransferase family functions in arabinogalactan-protein glycosylation, growth and development in Arabidopsis
- PMID: 26690932
- PMCID: PMC4687291
- DOI: 10.1186/s12870-015-0670-7
A small multigene hydroxyproline-O-galactosyltransferase family functions in arabinogalactan-protein glycosylation, growth and development in Arabidopsis
Abstract
Background: Arabinogalactan-proteins (AGPs) are ubiquitous components of cell walls throughout the plant kingdom and are extensively post translationally modified by conversion of proline to hydroxyproline (Hyp) and by addition of arabinogalactan polysaccharides (AG) to Hyp residues. AGPs are implicated to function in various aspects of plant growth and development, but the functional contributions of AGP glycans remain to be elucidated. Hyp glycosylation is initiated by the action of a set of Hyp-O-galactosyltransferase (Hyp-O-GALT) enzymes that remain to be fully characterized.
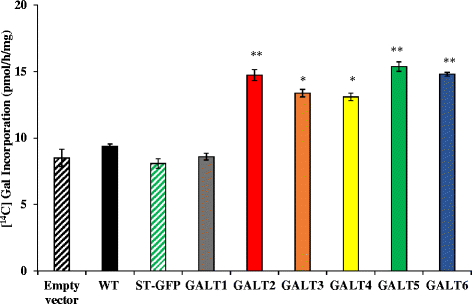
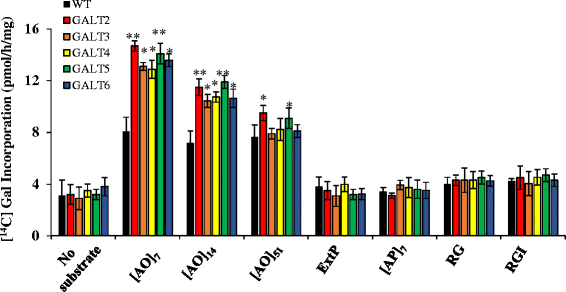
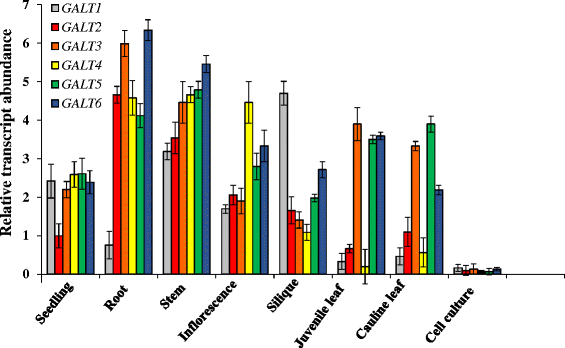
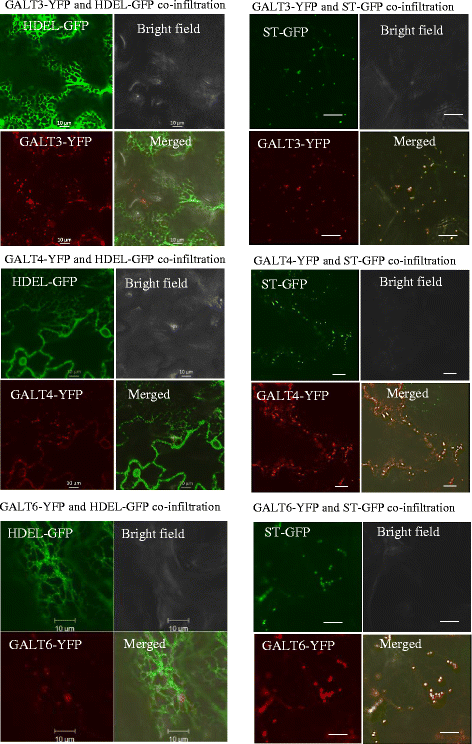
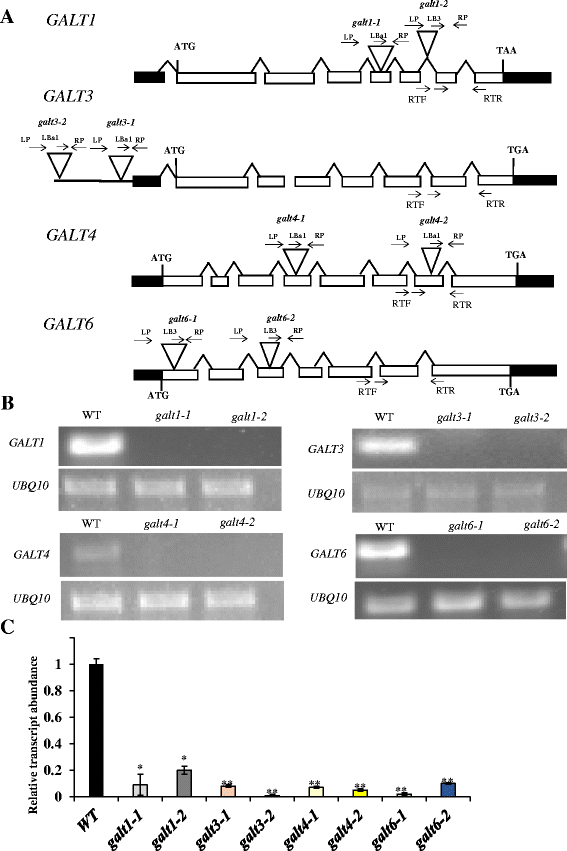
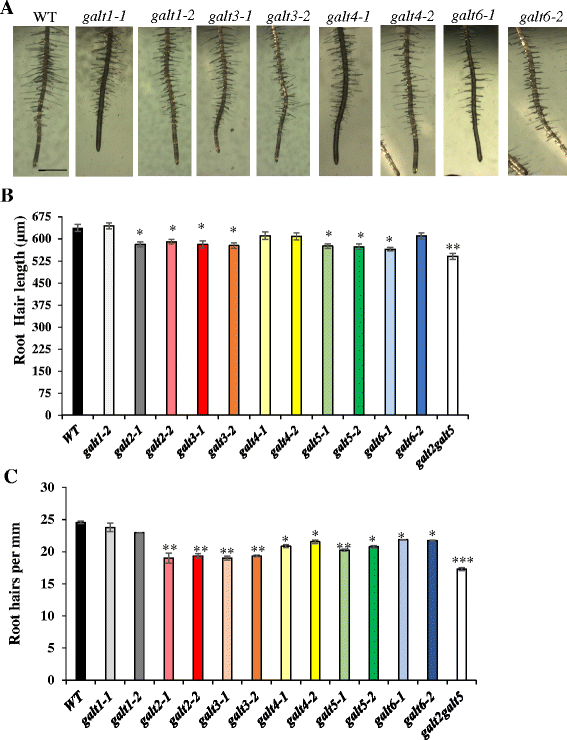
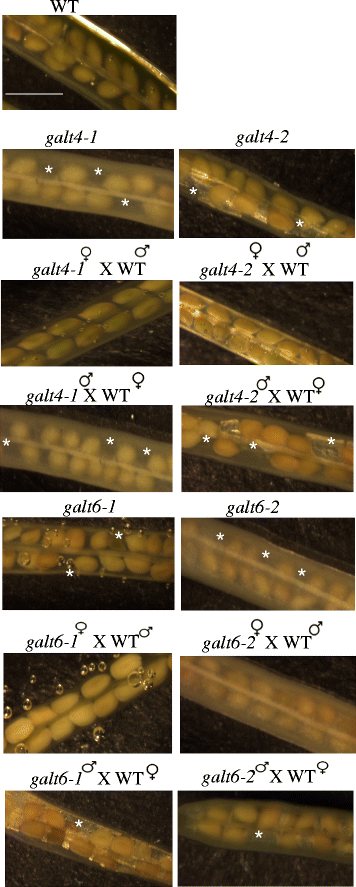
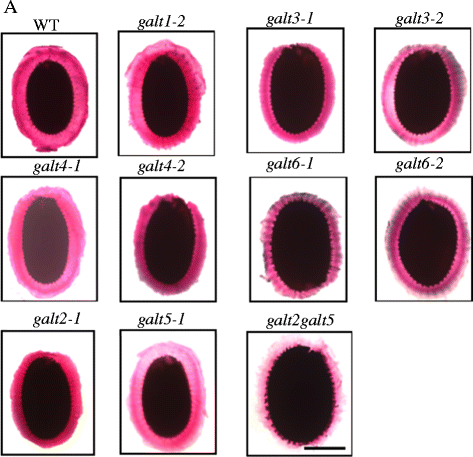
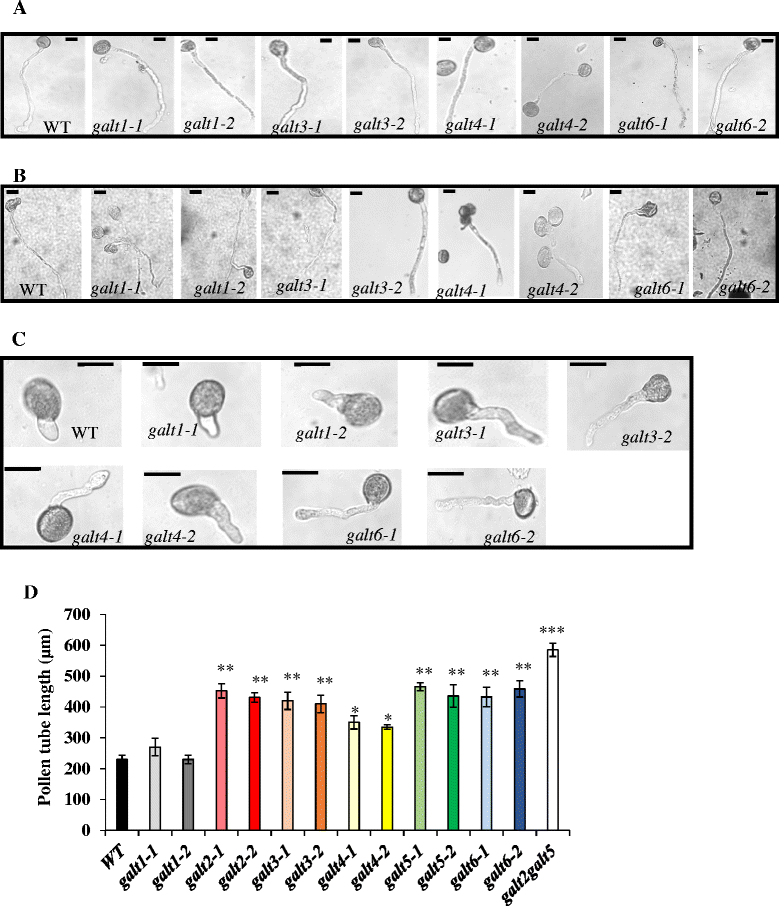
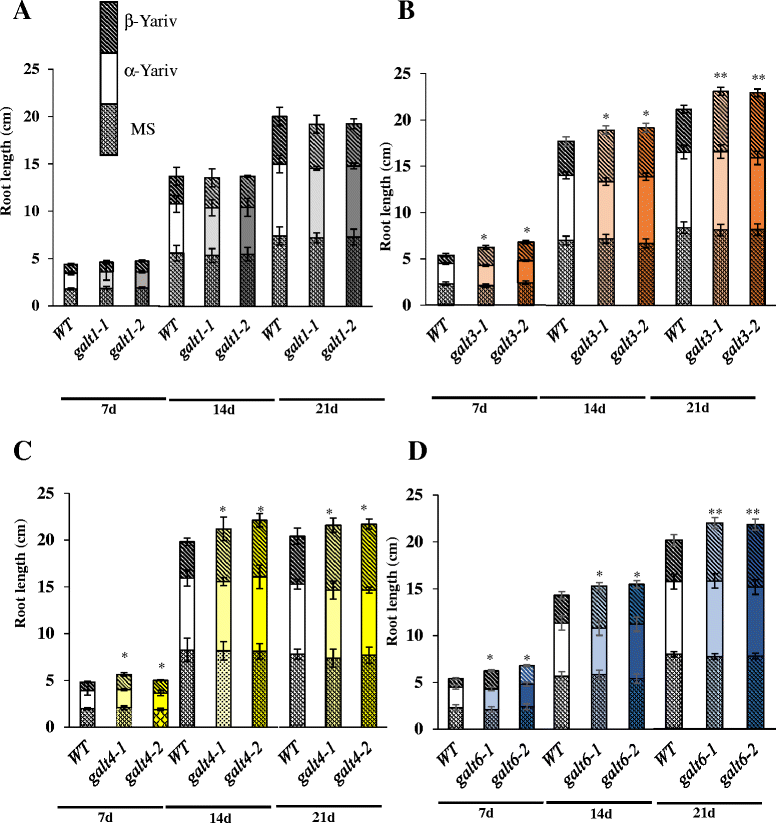
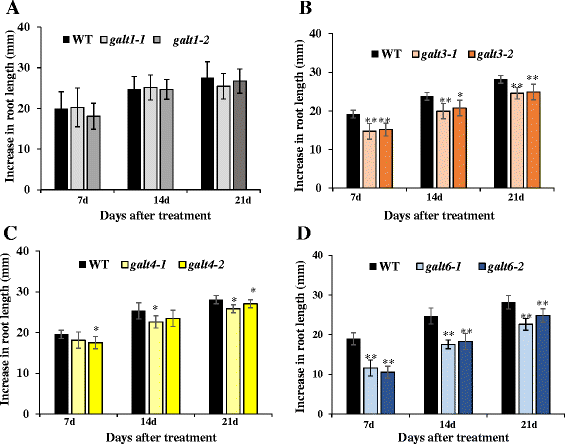
Results: Three members of the GT31 family (GALT3-At3g06440, GALT4-At1g27120, and GALT6-At5g62620) were identified as Hyp-O-GALT genes by heterologous expression in tobacco leaf epidermal cells and examined along with two previously characterized Hyp-O-GALT genes, GALT2 and GALT5. Transcript profiling by real-time PCR of these five Hyp-O-GALTs revealed overlapping but distinct expression patterns. Transiently expressed GALT3, GALT4 and GALT6 fluorescent protein fusions were localized within Golgi vesicles. Biochemical analysis of knock-out mutants for the five Hyp-O-GALT genes revealed significant reductions in both AGP-specific Hyp-O-GALT activity and β-Gal-Yariv precipitable AGPs. Further phenotypic analysis of these mutants demonstrated reduced root hair growth, reduced seed coat mucilage, reduced seed set, and accelerated leaf senescence. The mutants also displayed several conditional phenotypes, including impaired root growth, and defective anisotropic growth of root tips under salt stress, as well as less sensitivity to the growth inhibitory effects of β-Gal-Yariv reagent in roots and pollen tubes.
Conclusions: This study provides evidence that all five Hyp-O-GALT genes encode enzymes that catalyze the initial steps of AGP galactosylation and that AGP glycans play essential roles in both vegetative and reproductive plant growth.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

