The effects of MOTILIPERM on cisplatin induced testicular toxicity in Sprague-Dawley rats
- PMID: 26691229
- PMCID: PMC4683964
- DOI: 10.1186/s12935-015-0274-1
The effects of MOTILIPERM on cisplatin induced testicular toxicity in Sprague-Dawley rats
Abstract
Background: Cisplatin causes male infertility but the exact mechanism have not been clarified, yet. MOTILIPERM has been implicated in alleviation of infertility in Sprague-Dawley rats caused by cisplatin. We evaluated recovery effect of MOTILIPERM on cisplatin (CIS)-induced testicular toxicity in Sprague-Dawley rats.
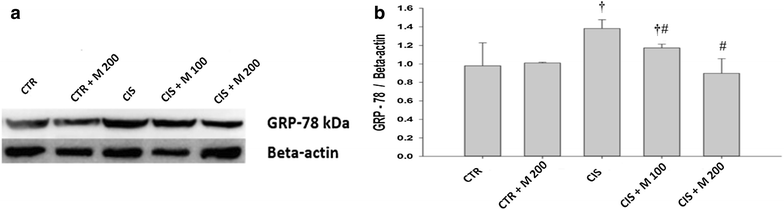
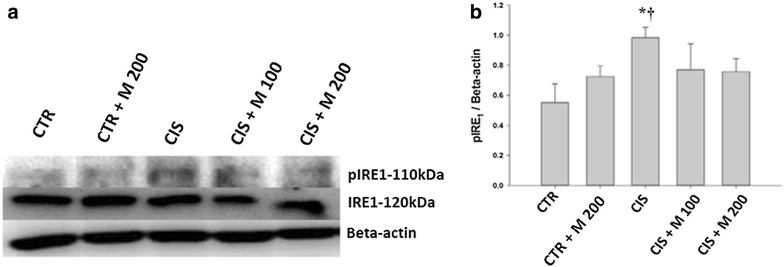
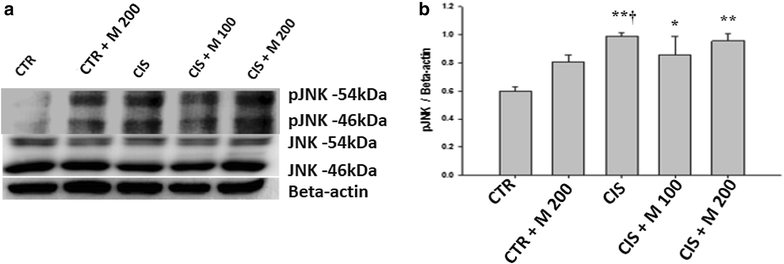
Methods: Five groups were included. The groups are control (CTR), CTR + MOTILIPERM 200 mg/kg/day per oral, CIS 10 mg/kg i.v., CIS 10 mg/kg + MOTILIPERM 100 mg/kg/day, CIS 10 mg/kg + MOTILIPERM 200 mg/kg/day. CIS 10 mg/kg i.v. single dose was given before 100 mg/kg, or 200 mg/kg MOTILIPERM per oral daily for 28 days. Body and genital organs weight, epididymis sperm count, sperm motility, sperm apoptosis, testosterone level, MDA of testis tissue, spermatogenic cell density, and Johnsen's score were evaluated. Steroidogenic acute regulatory (StAR) protein, and Glucose-regulated protein-78 (GRP-78), phosphorylated Inositol-Requiring Transmembrane Kinase/Endoribonuclease 1 (IRE1) and phosphorylated c-jun-N-terminal kinase (p-JNK) were quantitated by western blot to show its signaling pathway.
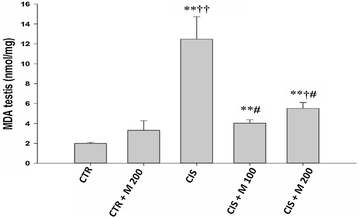
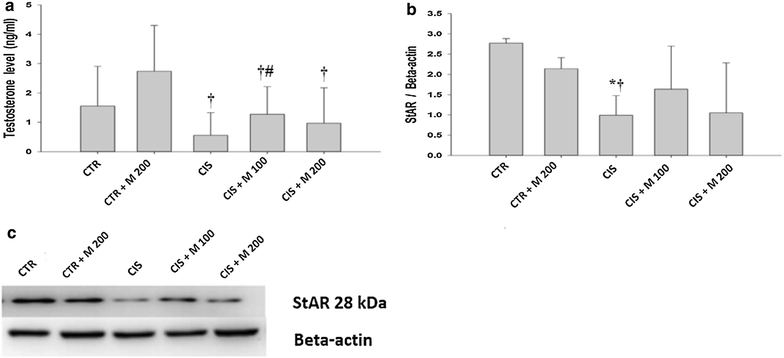
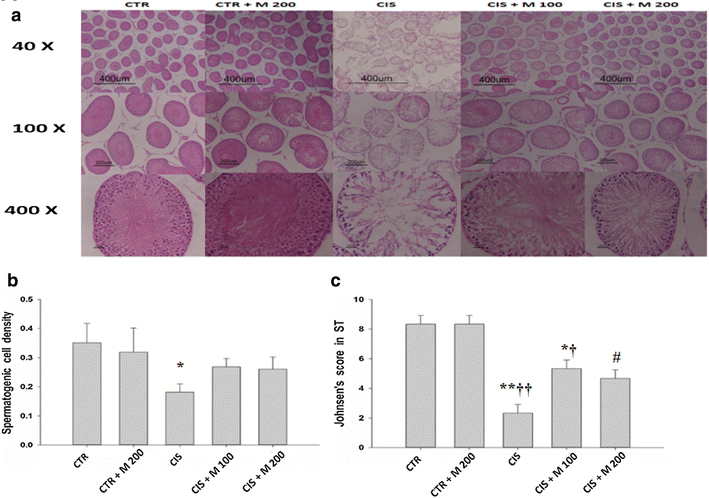
Results: The body weight was decreased significantly in CIS 10 mg/kg, CIS 10 mg/kg + MOTILIPERM 100 mg/kg/day, CIS 10 mg/kg + MOTILIPERM 200 mg/kg/day compared with CTR (p < 0.001) however, it was increased in CIS 10 mg/kg + MOTILIPERM 100 mg/kg/day, CIS 10 mg/kg + MOTILIPERM 200 mg/kg/day compared with CIS 10 mg/kg. The decreased weight of epididymis and prostate were increased significantly in CIS 10 mg/kg + MOTILIPERM 100 mg/kg/day compared with CIS 10 mg/kg. Sperm count, sperm motility, sperm apoptosis, MDA of testis tissue, spermatogenic cell density, Johnsen's score, and total testosterone were also significantly improved by MOTILIPERM treatment. The levels of decreased StAR protein was significantly improved by MOTILIPERM administration, increased GRP-78 protein p-IRE1and p-JNK was also significantly decreased with MOTILIPREM treatment.
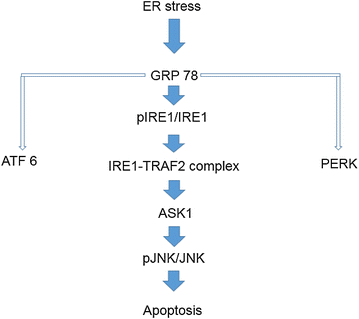
Conclusion: The MOTILIPERM could be an effective medicine to reduce the toxic effect caused ER stress by CIS in the testis.
Keywords: Cispaltin (CIS); Glucose-regulated protein-78 (GRP-78); MOTILIPERM; Phosphorylated c-jun-N-terminal kinase (p-JNK); Phosphorylated inositol-requiring transmembrane kinase/endoribonuclease 1 (IRE1); Spermatogenic cell denity; Steroidogenic acute regulatory (StAR) protein.
Figures
Similar articles
-
Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1α (p-IRE1α) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways.Pharm Biol. 2018 Dec;56(1):94-103. doi: 10.1080/13880209.2017.1421672. Pharm Biol. 2018. PMID: 29316840 Free PMC article.
-
MOTILIPERM Ameliorates Immobilization Stress-Induced Testicular Dysfunction via Inhibition of Oxidative Stress and Modulation of the Nrf2/HO-1 Pathway in SD Rats.Int J Mol Sci. 2020 Jul 3;21(13):4750. doi: 10.3390/ijms21134750. Int J Mol Sci. 2020. PMID: 32635386 Free PMC article.
-
Protective effect of DA-9401 in finasteride-induced apoptosis in rat testis: inositol requiring kinase 1 and c-Jun N-terminal kinase pathway.Drug Des Devel Ther. 2017 Oct 11;11:2969-2979. doi: 10.2147/DDDT.S140543. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 29066868 Free PMC article.
-
The Effect of Schisandra chinensis Baillon on Cross-Talk between Oxidative Stress, Endoplasmic Reticulum Stress, and Mitochondrial Signaling Pathway in Testes of Varicocele-Induced SD Rat.Int J Mol Sci. 2019 Nov 17;20(22):5785. doi: 10.3390/ijms20225785. Int J Mol Sci. 2019. PMID: 31744253 Free PMC article.
-
Gui-A-Gra Attenuates Testicular Dysfunction in Varicocele-Induced Rats via Oxidative Stress, ER Stress and Mitochondrial Apoptosis Pathway.Int J Mol Sci. 2020 Dec 3;21(23):9231. doi: 10.3390/ijms21239231. Int J Mol Sci. 2020. PMID: 33287403 Free PMC article.
Cited by
-
Effects of secretome on cisplatin-induced testicular dysfunction in rats.Vet World. 2018 Sep;11(9):1349-1356. doi: 10.14202/vetworld.2018.1349-1356. Epub 2018 Sep 29. Vet World. 2018. PMID: 30410245 Free PMC article.
-
Cisplatin Induces Apoptosis in Mouse Neonatal Testes Organ Culture.Int J Mol Sci. 2022 Nov 1;23(21):13360. doi: 10.3390/ijms232113360. Int J Mol Sci. 2022. PMID: 36362147 Free PMC article.
-
The protective effect of crocin on cisplatin-induced testicular impairment in rats.BMC Urol. 2021 Sep 1;21(1):117. doi: 10.1186/s12894-021-00889-2. BMC Urol. 2021. PMID: 34470647 Free PMC article.
-
Protective effect of MOTILIPERM in varicocele-induced oxidative injury in rat testis by activating phosphorylated inositol requiring kinase 1α (p-IRE1α) and phosphorylated c-Jun N-terminal kinase (p-JNK) pathways.Pharm Biol. 2018 Dec;56(1):94-103. doi: 10.1080/13880209.2017.1421672. Pharm Biol. 2018. PMID: 29316840 Free PMC article.
-
Dose-dependent effects of cisplatin on the severity of testicular injury in Sprague Dawley rats: reactive oxygen species and endoplasmic reticulum stress.Drug Des Devel Ther. 2016 Dec 12;10:3959-3968. doi: 10.2147/DDDT.S120014. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 28003740 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

