Disruption of Nrf2 Synergizes with High Glucose to Cause Heightened Myocardial Oxidative Stress and Severe Cardiomyopathy in Diabetic Mice
- PMID: 26691239
- PMCID: PMC4681446
- DOI: 10.4172/2155-6156.S7-002
Disruption of Nrf2 Synergizes with High Glucose to Cause Heightened Myocardial Oxidative Stress and Severe Cardiomyopathy in Diabetic Mice
Abstract
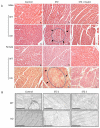
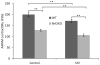
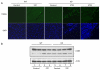
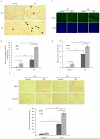
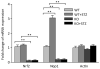
High glucose-induced oxidative stress is a major contributing mechanism to the development of diabetic cardiomyopathy. Nrf2 is an emerging critical regulator of cellular defense against oxidative damage. The role of Nrf2 in diabetic cardiomyopathy was investigated in vivo. Streptozotocin (STZ) induced diabetes in Nrf2 knockout (KO) mice that rapidly progressed to severe conditions with high mortality within two weeks of injection; whereas, in wild type (WT) mice, diabetes was less severe with no death. Severe myocardial lesions were observed in diabetic KO mice that had high, sublethal levels of blood glucose including: (a) irregular myocardial arrangements, myofibrillar discontinuation, and cell death; (b) reduced electron density, discontinuation of myocardial fibers, and mitochondrial damage; and (c) markedly reduced contractility of the cardiomyocytes to β-agonist stimulation. Parallel to severe cardiomyopathy, the diabetic KO hearts showed: (a) increased apoptosis as revealed by TUNEL and PARP1 cleavage assays; (b) infiltration of granulocytes and macrophages as well as fibrosis indicating robust inflammatory response; and (c) heightened oxidative stress as evidenced by increased levels of 8-hydroxydeoxyquanine, free malondialdehyde, and 3-nitrotyrosine. Increased oxidative stress in the KO hearts was attributed to decrease or loss of the basal and induced expression of Nrf2-dependent cytoprotective genes. Our findings demonstrate that loss of Nrf2 function synergizes with high glucose to cause heightened oxidative stress in the heart leading to severe diabetic cardiomyopathy.
Figures

Similar articles
-
Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes.J Mol Cell Cardiol. 2009 Jan;46(1):47-58. doi: 10.1016/j.yjmcc.2008.10.007. Epub 2008 Nov 1. J Mol Cell Cardiol. 2009. PMID: 19007787
-
Akap1 deficiency exacerbates diabetic cardiomyopathy in mice by NDUFS1-mediated mitochondrial dysfunction and apoptosis.Diabetologia. 2020 May;63(5):1072-1087. doi: 10.1007/s00125-020-05103-w. Epub 2020 Feb 19. Diabetologia. 2020. PMID: 32072193
-
Inhibiting microRNA-144 abates oxidative stress and reduces apoptosis in hearts of streptozotocin-induced diabetic mice.Cardiovasc Pathol. 2015 Nov-Dec;24(6):375-81. doi: 10.1016/j.carpath.2015.06.003. Epub 2015 Jun 19. Cardiovasc Pathol. 2015. PMID: 26164195
-
Current Status and Challenges of NRF2 as a Potential Therapeutic Target for Diabetic Cardiomyopathy.Int Heart J. 2019 May 30;60(3):512-520. doi: 10.1536/ihj.18-476. Epub 2019 Apr 10. Int Heart J. 2019. PMID: 30971629 Review.
-
Relationship between oxidative stress and nuclear factor-erythroid-2-related factor 2 signaling in diabetic cardiomyopathy (Review).Exp Ther Med. 2021 Jul;22(1):678. doi: 10.3892/etm.2021.10110. Epub 2021 Apr 25. Exp Ther Med. 2021. PMID: 33986843 Free PMC article. Review.
Cited by
-
Autophagy Inhibition Enables Nrf2 to Exaggerate the Progression of Diabetic Cardiomyopathy in Mice.Diabetes. 2020 Dec;69(12):2720-2734. doi: 10.2337/db19-1176. Epub 2020 Sep 18. Diabetes. 2020. PMID: 32948607 Free PMC article.
-
Pak2 Regulation of Nrf2 Serves as a Novel Signaling Nexus Linking ER Stress Response and Oxidative Stress in the Heart.Front Cardiovasc Med. 2022 Mar 8;9:851419. doi: 10.3389/fcvm.2022.851419. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 35350536 Free PMC article.
-
The Dark Side of Nrf2 in the Heart.Front Physiol. 2020 Jul 9;11:722. doi: 10.3389/fphys.2020.00722. eCollection 2020. Front Physiol. 2020. PMID: 32733266 Free PMC article. Review.
-
Ferroptosis: A New Mechanism in Diabetic Cardiomyopathy.Int J Med Sci. 2024 Jan 21;21(4):612-622. doi: 10.7150/ijms.88476. eCollection 2024. Int J Med Sci. 2024. PMID: 38464828 Free PMC article. Review.
-
DNA Damage: A Main Determinant of Vascular Aging.Int J Mol Sci. 2016 May 18;17(5):748. doi: 10.3390/ijms17050748. Int J Mol Sci. 2016. PMID: 27213333 Free PMC article. Review.
References
-
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. - PubMed
-
- Murarka S, Movahed MR. Diabetic cardiomyopathy. J Card Fail. 2010;16:971–979. - PubMed
-
- Khavandi K, Khavandi A, Asghar O, Greenstein A, Withers S, et al. Diabetic cardiomyopathy--a distinct disease? Best Pract Res Clin Endocrinol Metab. 2009;23:347–360. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
