Interventions for central serous chorioretinopathy: a network meta-analysis
- PMID: 26691378
- PMCID: PMC5030073
- DOI: 10.1002/14651858.CD011841.pub2
Interventions for central serous chorioretinopathy: a network meta-analysis
Update in
-
Interventions for central serous chorioretinopathy: a network meta-analysis.Cochrane Database Syst Rev. 2025 Jun 16;6(6):CD011841. doi: 10.1002/14651858.CD011841.pub3. Cochrane Database Syst Rev. 2025. PMID: 40522203
Abstract
Background: Central serous chorioretinopathy (CSC) is characterized by serous detachment of the neural retina with dysfunction of the choroid and retinal pigment epithelium (RPE). The effects on the retina are usually self limited, although some people are left with irreversible vision loss due to progressive and permanent photoreceptor damage or RPE atrophy. There have been a variety of interventions used in CSC, including, but not limited to, laser treatment, photodynamic therapy (PDT), and intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) agents. However, it is not known whether these or other treatments offer significant advantages over observation or other interventions. At present there is no evidence-based consensus on the management of CSC. Due in large part to the propensity for CSC to resolve spontaneously or to follow a waxing and waning course, the most common initial approach to treatment is observation. It remains unclear whether this is the best approach with regard to safety and efficacy.
Objectives: To compare the relative effectiveness of interventions for central serous chorioretinopathy.
Search methods: We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2015, Issue 9), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to February 2014), EMBASE (January 1980 to October 2015), the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 5 October 2015.
Selection criteria: Randomized controlled trials (RCTs) that compared any intervention for CSC with any other intervention for CSC or control.
Data collection and analysis: Two review authors independently selected studies and extracted data. We pooled data from all studies using a fixed-effect model. For interventions applied to the eye (i.e. not systemic interventions), we synthesized direct and indirect evidence in a network meta-analysis model.
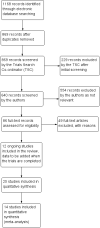
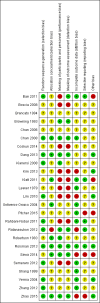
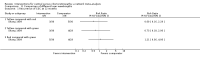
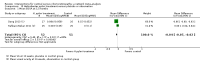
Main results: We included 25 studies with 1098 participants (1098 eyes) and follow-up from 16 weeks to 12 years. Studies were conducted in Europe, North and South America, Middle East, and Asia. The trials were small (most trials enrolled fewer than 50 participants) and poorly reported; often it was unclear whether key aspects of the trial, such as allocation concealment, had been done. A substantial proportion of the trials were not masked.The studies considered a variety of treatments: anti-VEGF (ranibizumab, bevacizumab), PDT (full-dose, half-dose, 30%, low-fluence), laser treatment (argon, krypton and micropulse laser), beta-blockers, carbonic anhydrase inhibitors, Helicobactor pylori treatment, and nutritional supplements (Icaps, lutein); there were only one or two trials contributing data for each comparison. We downgraded for risk of bias and imprecision for most analyses, reflecting study limitations and imprecise estimates. Network meta-analysis (as planned in our protocol) did not help to resolve this uncertainty due to a lack of trials, and problems with intransitivity, particularly with respect to acute or chronic CSC.Low quality evidence from two trials suggested little difference in the effect of anti-VEGF (ranibizumab or bevacizumab) or observation on change in visual acuity at six months in acute CSC (mean difference (MD) 0.01 LogMAR (logarithm of the minimal angle of resolution), 95% confidence interval (CI) -0.02 to 0.03; 64 participants). CSC had resolved in all participants by six months. There were no significant adverse effects noted.Low quality evidence from one study (58 participants) suggested that half-dose PDT treatment of acute CSC probably results in a small improvement in vision (MD -0.10 logMAR, 95% CI -0.18 to -0.02), less recurrence (risk ratio (RR) 0.10, 95% CI 0.01 to 0.81) and less persistent CSC (RR 0.12, 95% CI 0.01 to 1.02) at 12 months compared to sham treatment. There were no significant adverse events noted.Low quality evidence from two trials (56 participants) comparing anti-VEGF to low-fluence PDT in chronic CSC found little evidence for any difference in visual acuity at 12 months (MD 0.03 logMAR, 95% CI -0.08 to 0.15). There was some evidence that more people in the anti-VEGF group had recurrent CSC compared to people treated with PDT but, due to inconsistency between trials, it was difficult to estimate an effect. More people in the anti-VEGF group had persistent CSC at 12 months (RR 6.19, 95% CI 1.61 to 23.81; 34 participants).Two small trials of micropulse laser, one in people with acute CSC and one in people with chronic CSC, provided low quality evidence that laser treatment may lead to better visual acuity (MD -0.20 logMAR, 95% CI -0.30 to -0.11; 45 participants). There were no significant adverse effects noted.Other comparisons were largely inconclusive.We identified 12 ongoing trials covering the following interventions: aflibercept and eplerenone in acute CSC; spironolactone, eplerenone, lutein, PDT, and micropulse laser in chronic CSC; and micropulse laser and oral mifepristone in two trials where type of CSC not clearly specified.
Authors' conclusions: CSC remains an enigmatic condition in large part due to a natural history of spontaneous improvement in a high proportion of people and also because no single treatment has provided overwhelming evidence of efficacy in published RCTs. While a number of interventions have been proposed as potentially efficacious, the quality of study design, execution of the study and the relatively small number of participants enrolled and followed to revealing endpoints limits the utility of existing data. It is not clear whether there is a clinically important benefit to treating acute CSC which often resolves spontaneously as part of its natural history. RCTs comparing individual treatments to the natural history would be valuable in identifying potential treatment groups for head-to-head comparison. Of the interventions studied to date, PDT or micropulse laser treatment appear the most promising for study in future trials.
Conflict of interest statement
DECLARATIONS OF INTEREST None known.
Figures



















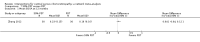










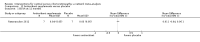


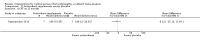





References
References to studies included in this review
Bae 2011 {published data only}
-
- Bae SH, Heo J, Kim C, Kim TW, Shin JY, Lee JY, et al. Low‐fluence photodynamic therapy versus ranibizumab for chronic central serous chorioretinopathy: one‐year results of a randomized trial. Ophthalmology 2014;121(2):558‐65. - PubMed
-
- Bae SH, Heo JW, Kim C, Kim TW, Lee JY, Song SJ, et al. A randomized pilot study of low‐fluence photodynamic therapy versus intravitreal ranibizumab for chronic central serous chorioretinopathy. American Journal of Ophthalmology 2011;152(5):784‐92. - PubMed
Boscia 2008 {published data only}
-
- Boscia F, Michele R, Antonio L, Nicola C, Claudio F, Salvatore F, et al. Low fluence photodynamic therapy in chronic central serous chorioretinopathy: blind randomized clinical trial of efficacy and safety. The Macula Society 2008:118.
-
- Boscia F, Reibaldi M, Longo A, Faro S, Uva M, Cardascia N, et al. Low fluence photodynamic therapy in chronic central serous chorioretinopathy: blind randomised clinical trial of efficacy and safety. Investigative Ophthalmology and Visual Science 2008:ARVO E‐abstract 3278.
Brancato 1994 {published data only}
-
- Brancato R, Bandello F. Treatment of central serous chorioretinopathy with beta‐blockers and calcium antagonists. The Macula Society 1994:114.
Browning 1993 {published data only}
-
- Browning DJ. Nadolol in the treatment of central serous retinopathy. American Journal of Ophthalmology 1993; Vol. 116, issue 6:770‐1. - PubMed
Chan 2006 {published data only}
-
- Chan PS, Uy HS, Salvosa F, Silva PA. Low dose transpupillary thermotherapy for the treatment of central serous chorioretinopathy. American Academy of Ophthalmology 2006:290.
Chan 2008 {published data only}
-
- Chan WM, Lai TY, Lai RY, Liu DT, Lam DS. Half‐dose verteporfin photodynamic therapy for acute central serous chorioretinopathy: one‐year results of a randomized controlled trial. Ophthalmology 2008;115(10):1756‐65. - PubMed
-
- Wu ZH, Lai RY, Yip YW, Chan WM, Lam DS, Lai TY. Improvement in multifocal electroretinography after half‐dose verteporfin photodynamic therapy for central serous chorioretinopathy: a randomized placebo‐controlled trial. Retina 2011;31(7):1378‐86. - PubMed
Coskun 2014 {published data only}
-
- Coskun E, Gurler B, Erbagci I. Combined half dose photodynamic therapy with verteporfin and intravitreal bevacizumab for chronic central serous chorioretinopathy. Ophthalmologica 2014;232:56.
Dang 2013 {published data only}
Kianersi 2008 {published data only}
-
- Kianersi F, Fesharaki F. Effects of propranolol in patients with central serous chorioretinopathy. Journal of Research in Medical Sciences 2008;13(3):103‐7.
Kim 2013 {published data only}
-
- Kim M, Lee SC, Lee SJ. Intravitreal ranibizumab for acute central serous chorioretinopathy. Ophthalmologica 2013;229(3):152‐7. - PubMed
Klatt 2011 {published data only}
-
- Klatt C, Saeger M, Oppermann T, Pörksen, E, Treumer F, Hillenkamp J, et al. Selective retina therapy for acute central serous chorioretinopathy. British Journal of Ophthalmology 2011; Vol. 95, issue 1:83‐8. - PubMed
Leaver 1979 {published data only}
-
- Ficker L, Vafidis G, While A, Leaver P. Long term results of treatment of central serous retinopathy ‐ a preliminary report. Transactions of the Ophthalmological Societies of the United Kingdom 1986;105(Pt 4):473‐5. - PubMed
Lim 2010 {published data only}
Ontiveros‐Orozco 2004 {published data only}
-
- Ontiveros‐Orozco I, Garcia‐Franco R, Levine‐Berebichez A, Celis‐Suazo B, Rojas‐Juarez S. Topical brinzolamide for the treatment of idiopathic central serous chorioretinopathy. Investigative Ophthalmology and Visual Science 2004; Vol. 45:ARVO E‐abstract 529.
-
- Ontiveros‐Orozco I, García‐Franco R, Levine‐Berevichez A, López‐Star EM, Rojas‐Juárez S, Celis‐Suazo B, et al. Brinzolamide for topical treatment coroidorretinopatía idiopathic central serous [Brinzolamida tópica para el tratamiento de lacoroidorretinopatía serosa central idiopática]. Revista Mexicana de Oftalmología 2006;80(3):132‐7.
Pitcher 2015 {published data only}
-
- Pitcher JD 3rd, Witkin AJ, DeCroos FC, Ho AC. A prospective pilot study of intravitreal aflibercept for the treatment of chronic central serous chorioretinopathy: the CONTAIN study. British Journal of Ophthalmology 2015;99(6):848‐52. - PubMed
Rahbani‐Nobar 2011 {published data only}
Ratanasukon 2012 {published data only}
Robertson 1983 {published data only}
-
- Robertson DM, Ilstrup D. Direct, indirect, and sham laser photocoagulation in the management of central serous chorioretinopathy. American Journal of Ophthalmology 1983;95(4):457‐66. - PubMed
Roisman 2013 {published data only}
-
- Roisman L, Magalhaes FP, Lavinsky D, Moraes N, Hirai FE, Cardillo JA, et al. Micropulse diode laser treatment for chronic central serous chorioretinopathy: a randomized pilot trial. Ophthalmic Surgery Lasers and Imaging 2013;44(5):465‐70. - PubMed
Sawa 2014 {published data only}
-
- Sawa M, Gomi F, Hara C, Nishida K. Effects of a lutein supplement on the plasma lutein concentration and macular pigment in patients with central serous chorioretinopathy. Investigative Ophthalmology and Visual Science 2014; Vol. 55, issue 8:5238‐44. - PubMed
Semeraro 2012 {published data only}
-
- Semeraro F, Romano MR, Danzi P, Morescalchi F, Costagliola C. Intravitreal bevacizumab versus low‐fluence photodynamic therapy for treatment of chronic central serous chorioretinopathy. Japanese Journal of Ophthalmology 2012;56(6):608‐12. - PubMed
Shang 1999 {published data only}
-
- Shang Q, Liu C, Wei S, Shi F, Yang A. Wavelength selection in management of central serous chorioretinopathy. Chinese Journal of Ophthalmology 1999;35(6):413‐5. - PubMed
Verma 2004 {published data only}
Zhang 2012 {published data only}
-
- Zhang YL, You ZP, Wang CY. Different doses of verteporfin photodynamic therapy for central exudative chorioretinopathy. Chinese Journal of Experimental Ophthalmology 2012;30(11):1030‐5.
Zhao 2015 {published data only}
-
- Zhao M, Zhang F, Chen Y, Dai H, Qu J, Dong C, et al. A 50% vs 30% dose of verteporfin (photodynamic therapy) for acute central serous chorioretinopathy: one‐year results of a randomized clinical trial. JAMA Ophthalmology 2015;133(3):333‐40. - PubMed
References to studies excluded from this review
Ainiwaer 2014 {published data only}
-
- Ainiwaer K, Xiong LJ. Efficacy of compound Xueshuantong combined laser therapy on central serous chorioretinopathy. International Eye Science 2014;14(10):1841‐3.
Arevalo 2013 {published data only}
-
- Arevalo JF, Espinoza JV. Combined photodynamic therapy with verteporfin and intravitreal anti‐vascular endothelial growth factor therapy for chronic central serous chorioretinopathy. Graefes Archive for Clinical and Experimental Ophthalmology 2013;251(1):403‐4. - PubMed
Aydin 2013 {published data only}
-
- Aydin E. The efficacy of intravitreal bevacizumab for acute central serous chorioretinopathy. Journal of Ocular Pharmacology and Therapeutics 2013;29(1):10‐3. - PubMed
Beger 2012 {published data only}
-
- Beger I, Koss M J, Koch F. Treatment of central serous chorioretinopathy: micropulse photocoagulation versus bevacizumab. Der Ophthalmologe 2012;109(12):1224‐32. - PubMed
Behnia 2013 {published data only}
-
- Behnia M, Khabazkhoob M, Aliakbari S, Abadi A E, Hashemi H, Pourvahidi P. Improvement in visual acuity and contrast sensitivity in patients with central serous chorioretinopathy after macular subthreshold laser therapy. Retina 2013;33(2):324‐8. - PubMed
Bi 2000 {published data only}
-
- Bi HS, Liu L, Wang XR, Yang MY. Treatment of central serous chorioretinopathy with krypton yellow and green laser photocoagulation. Ophthalmology 2000;9(3):166‐8.
Boscia 2007 {published data only}
-
- Boscia F, Caradscia N, Furino C, Dammacco R, Sborgia G, Reibaldi M. Photodynamic therapy with low fluence PDT for chronic central serous chorioretinopathy: a short term pilot study. Investigative Ophthalmology and Visual Science 2007; Vol. 48:ARVO E‐Abstract 4151.
Bruha 1972 {published data only}
-
- Bruha H. Therapy of the so‐called chorioretinitis centralis serosa with the laser‐coagulator. Klinische Monatsblatter fur Augenheilkunde 1972;160(3):340‐6. - PubMed
Cervera 2008 {published data only}
-
- Cervera E, Montero J, Torralba C, Palomares P, Hernandez M. Low dose photodynamic therapy for chronic central serous chorioretinopathy. Archivos de la Sociedad Espanola de Oftalmologia 2008;83(9):525‐6. - PubMed
Chrapek 2015 {published data only}
-
- Chrapek O, Jirkova B, Kandrnal V, Rehak J, Sin M. Treatment of central serous chorioretinopathy with beta‐blocker metipranolol. Biomedical Papers of the Medical Faculty of Palacky University in Olomouc, Czech Republic 2015;159(1):120‐3. - PubMed
Demirel 2014 {published data only}
-
- Demirel S, Ozmert E, Batioglu F. The comparison of reduced‐fluence photodynamic therapy with 577 nm yellow‐wavelength pattern micropulse laser therapy in cases with chronic central serous chorioretinopathy: short term results. Ophthalmologica 2014;232(Suppl 2):60.
Di 2013 {published data only}
-
- Di Y, Gui DM, Yang HW, Chen XL. Changes in visual function before and after laser photocoagulation of central serous chorioretinopathy. International Eye Science 2013;13(2):336‐8.
Earl 2014 {published data only}
-
- Earl JB, Lee CS, Yom V, Stavern GP, Abuattieh M, Chin‐Yee D, et al. Visual cycle suppression via patching in central serous chorioretinopathy. Ophthalmology 2014;121(12):2502‐4. - PubMed
Fang 2013 {published data only}
-
- Fang TB, Yan H, Xu ZR. Argon laser treatment of central serous chorioretinopathy. International Eye Science 2013;13(4):740‐2.
Feily 2009 {published data only}
-
- Feily A, Namazi MR. The potential utility of coumarin and horse chestnut extract for treatment of central serous chorioretinopathy. Nigerian Journal of Medicine 2009;18(4):434‐5. - PubMed
Haas 2004 {published data only}
-
- Haas A, Weger M, Furschuss‐Wolff P, Bachernegg M. Photodynamic therapy in patients with central serous chorioretinopathy. Spektrum der Augenheilkunde 2004;18(2):104.
Heinrich 1974 {published data only}
-
- Heinrich MR. Central serous retinopathy and alpha‐blockaders. Bulletin des Sociétés d'Ophtalmologie de France 1974;74(5‐6):681‐3. - PubMed
Huang 2006 {published data only}
-
- Huang Y, Wang Z, Zhang M. Clinical effectiveness of lecithin‐bound iodine on central serous chorioretinopathy following laser photocoagulation. Chinese Ophthalmic Research 2006;24(5):546‐8.
Khosla 1997 {published data only}
-
- Khosla PK, Rana SS, Tewari HK, Azad RU, Talwar D. Evaluation of visual function following argon laser photocoagulation in central serous retinopathy. Ophthalmic Surgery and Lasers 1997;28(8):693‐7. - PubMed
Koss 2012 {published data only}
Kurimoto 1969 {published data only}
-
- Kurimoto S, Fukunaga K, Momomura K. Effect of adenosine monophosphate on retinitis centralis. Nihon Ganka Kiyo 1969;20(8):841‐50. - PubMed
Lee 2011 {published data only}
-
- Lee JY, Chae JB, Yang SJ, Kim JG, Yoon YH. Intravitreal bevacizumab versus the conventional protocol of photodynamic therapy for treatment of chronic central serous chorioretinopathy. Acta Opthalmologica 2011;89(3):e293‐4. - PubMed
Li 2010 {published data only}
-
- Li XJ, Zhang JS. Intravitreal bevacizumab injection for chronic central serous chorioretinopathy. Chinese Medical Journal 2010;123(15):2145‐7. - PubMed
Lim 2011 {published data only}
-
- Lim JW, Kang SW, Kim YT, Chung SE, Lee SW. Comparative study of patients with central serous chorioretinopathy undergoing focal laser photocoagulation or photodynamic therapy. British Journal of Ophthalmology 2011;95(4):514‐7. - PubMed
Liu 2009 {published data only}
-
- Liu ZC, Li R. 532 Laser photocoagulation for the treatment of central serous chorioretinopathy. International Journal of Ophthalmology 2009;9(12):2451‐2.
Long 2011 {published data only}
-
- Long F, Cao SJ, Wang LP, Qi F, Xiu WW. Study in krypton laser combined with compound anisodine for central serous chorioretinopathy. International Journal of Ophthalmology 2011;11(2):330‐1.
Lyons 1977 {published data only}
-
- Lyons DE. Conservative management of central serous retinopathy. Transactions of the Ophthalmological Societies of the United Kingdom 1977;97(1):214‐6. - PubMed
Mackowiakowa 1987 {published data only}
-
- Mackowiakowa A, Pecoldowa K, Szwarcowa C. Comparison of late results of conservative treatment and laser coagulation of central serous chorioretinopathy [Porownanie wynikow odleglych po leczeniu zachowawczym i laserokoagulacji choroidoretinopatii surowiczej srodkowej]. Klinika Oczna 1987;89(4):162‐4. - PubMed
Miyashita 1971 {published data only}
-
- Miyashita S, Sakai M, Kanai A, Hayashi E. Successful treatment of central serous retinopathy by tranexamic acid injection. Nippon Ganka Gakkai Zasshi 1971;75:603‐8. - PubMed
NCT01256580 {unpublished data only}
-
- NCT01256580. Pilot study of intravitreal bevacizumab vs. combination therapy for choroidal neovascularization secondary to causes other than age‐related macular degeneration. clinicaltrials.gov/ct2/show/NCT01256580 (accessed 16 October 2015).
NCT01585441 {unpublished data only}
-
- NCT01585441. Phase II, randomized, placebo‐controlled study for the evaluation of finasteride in the treatment of chronic central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01585441 (accessed 16 October 2015).
Novak 1987 {published data only}
-
- Novak MA, Singerman LJ, Rice TA. Krypton and argon laser photocoagulation for central serous chorioretinopathy. Retina 1987;7(3):162‐9. - PubMed
Okamoto 2015 {published data only}
-
- Okamoto M, Matsuura T, Ogata N. Choroidal thickness and choroidal blood flow after intravitreal bevacizumab injection in eyes with central serous chorioretinopathy. Ophthalmic Surgery Lasers and Imaging Retina 2015; Vol. 46, issue 1:25‐32. - PubMed
Ozdemir 2014 {published data only}
-
- Ozdemir O, Erol MK. Morphologic changes and visual outcomes in resolved central serous chorioretinopathy treated with ranibizumab. Cutaneous and Ocular Toxicology 2014;33(2):122‐6. - PubMed
Peng 2010 {published data only}
-
- Peng QH, Peng J, Wu QL, Tan HY. Clinical study of method of activating blood and diuresis on central serous. International Journal of Ophthalmology 2010;10(7):1284‐6.
Radian 1984 {published data only}
-
- Radian AB, Mocanu C. Enzymatic treatment of central serous retinopathy. Revista de Chirurgie, Oncologie, Radiologie, ORL, Oftalmologie, Stomatologie. Oftalmologie 1984;28(4):299‐300. - PubMed
Sanchez‐Pacheco 2010 {published data only}
-
- Sanchez‐Pacheco Tardon L, Pardo Saiz A, Nebot Martinez J, Jornet Montana S. Mifepristone as a treatment alternative to chronic serous chorioretinopathy. Farmacia Hospitalaria 2010;34(1):44‐5. - PubMed
Takagi 1965 {published data only}
-
- Takagi M. Statistic studies of retinitis centralis. Nippon Ganka Kiyo 1965;16(7):420‐3. - PubMed
Tewari 1986 {published data only}
-
- Tewari HK, Azad R, Khosla PK, Kumar A. Argon laser photocoagulation in treatment of central serous retinopathy ‐ a prospective randomized study. Indian Journal of Ophthalmology 1986;34:251‐3. - PubMed
Wang 2009a {published data only}
-
- Wang XX. Clinical analysis of argon laser in treating central serous chorioretinopathy. International Journal of Ophthalmology 2009;9(2):384‐5.
Wang 2009b {published data only}
-
- Wang TT, Xu GX. Clinical observation of alliance application of compound anisodine and joletion in the treatment of central serous chorioretinopathy. International Journal of Ophthalmology 2009;9(6):1169‐71.
Watzke 1974 {published data only}
-
- Watzke RC, Burton TC, Leaverton P. Ruby laser photocoagulation therapy of central serous retinopathy. A preliminary report. Modern Problems in Ophthalmology 1974;12(0):242‐6. - PubMed
Watzke 1979 {published data only}
-
- Watzke RC, Burton TC, Woolson RF. Direct and indirect laser photocoagulation of central serous choroidopathy. American Journal of Ophthalmology 1979;88(5):914‐8. - PubMed
Wu 2010 {published data only}
-
- Wu SJ, Zeng ZC. Clinical observation of Jolethin combined with argon laser for central serous chorioretinopathy. International Journal of Ophthalmology 2010;10(12):2295‐7.
Xu 2013 {published data only}
-
- Xu B. Efficacy comparison of 577nm micro‐pulse laser and drug in the treatment of central serous chorioretinopathy. International Eye Science 2013;13(8):1688‐90.
Xu 2014 {published data only}
-
- Xu JF, Chen KC. Treatment of juxtafoveal central serous chorioretinopathy by compound anisodine injection. International Eye Science 2014;14(4):701‐3.
Ye 2013 {published data only}
-
- Ye J, Yang XY, Zeng S. Compound Xueshuantong capsule joint laser treating central serous chorioretinopathy. International Eye Science 2013;13(6):1160‐2.
Zhang 2014 {published data only}
-
- Zhang J, Xue GM, Zhang QY, Cao Y. Clinical observation of laser treatment for central serous chorioretinopathy choroidal diseases and study on relationship between visual acuity with leakage location. International Eye Science 2014;14(5):872‐4.
Zheng 2013 {published data only}
-
- Zheng TF, Qin YY. Hyperbaric oxygen therapy combined with jolethin in treating central serous chorioretinopathy. International Eye Science 2013;13(10):2124‐6.
References to ongoing studies
EUCTR2009‐017959‐98‐NL {unpublished data only}
-
- EUCTR2009‐017959‐98‐NL. Early treatment of patients with central serous retinopathy: a randomized controlled trial ‐ CSR & PDT. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2009‐017959‐98‐NL (accessed 16 October 2015).
JPRN‐UMIN000005372 {unpublished data only}
-
- JPRN‐UMIN000005372. Study on the effects of supplements containing lutein on spontaneous resolution in eyes with chronic central serous chorioretinopathy. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000005372 (accessed 16 October 2015).
NCT01019668 {unpublished data only}
-
- NCT01019668. Central serous chorioretinopathy treated by modified photodynamic therapy. clinicaltrials.gov/ct2/show/NCT01019668 (accessed 16 October 2015).
NCT01552044 {unpublished data only}
-
- NCT01552044. Effect of spironolactone in treating chronic non‐resolutive central serous chorioretinitis [Evaluation de la spironolactone dans le traitement des choriorétinites séreuses centrales non résolutives à trois mois]. clinicaltrials.gov/ct2/show/NCT01552044 (accessed 16 October 2015).
NCT01630863 {unpublished data only}
-
- NCT01630863. The safe effective light dose of photodynamic therapy for chronic central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01630863 (accessed 16 October 2015).
NCT01797861 {unpublished data only}
-
- Breukink MB, Downes SM, Querques G, Dijk EH, Hollander AI, Blanco‐Garavito R, et al. Comparing half‐dose photodynamic therapy with high‐density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (the PLACE trial): study protocol for a randomized controlled trial. TRIALS 2015;16:419. - PMC - PubMed
-
- NCT01797861. A prospective randomized controlled multicentre trial comparing half‐dose photodynamic therapy (PDT) with high‐density subthreshold micropulse laser treatment in patients with chronic central serous chorioretinopathy (CSC). clinicaltrials.gov/ct2/show/NCT01797861 (accessed 16 October 2015).
NCT01971190 {unpublished data only}
-
- NCT01971190. Efficacy and safety of intravitreal aflibercept injection for subacute central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01971190 (accessed 16 October 2015).
NCT01982383 {unpublished data only}
-
- NCT01982383. A prospective study of the use of micropulse 577nm laser treatment in central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01982383 (accessed 16 October 2015).
NCT01990677 {unpublished data only}
-
- NCT01990677. Eplerenone for the treatment of central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT01990677 (accessed 16 October 2015).
NCT02153125 {unpublished data only}
-
- NCT02153125. Eplerenone for the treatment of chronic central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT02153125 (accessed 16 October 2015).
NCT02215330 {unpublished data only}
-
- NCT02215330. A randomized, double‐masked, placebo controlled study of the beneficial effects of eplerenone on central serous chorioretinopathy. clinicaltrials.gov/ct2/show/NCT02215330 (accessed 16 October 2015).
NCT02354170 {unpublished data only}
-
- NCT02354170. Short‐term oral mifepristone for central serous chorioretinopathy. A placebo‐controlled dose ranging study of mifepristone in the treatment of CSC (STOMP‐CSC). clinicaltrials.gov/ct2/show/NCT02354170 (accessed 16 October 2015).
Additional references
Ahad 2006
-
- Ahad MA, Chua CN, Evans NM. Central serous chorioretinopathy associated with testosterone therapy. Eye 2006;20(4):503‐5. - PubMed
Baran 2005
-
- Baran NV, Gürlü VP, Esgin H. Long‐term macular function in eyes with central serous chorioretinopathy. Clinical and Experimental Ophthalmology 2005;33(4):369‐72. - PubMed
Bousquet 2013
-
- Bousquet E, Beydoun T, Zhao M, Hassan L, Offret O, Behar‐Cohen F. Mineralocorticoid receptor antagonism in the treatment of chronic central serous chorioretinopathy: a pilot study. Retina 2013;33(10):2096‐102. - PubMed
Bouzas 2002
-
- Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Survey of Ophthalmology 2002;47(5):431‐48. - PubMed
Bucher 1997
-
- Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta‐analysis of randomized controlled trials. Journal of Clinical Epidemiology 1997;50:683‐91. - PubMed
Cardillo Piccolino 2003
-
- Cardillo Piccolino F, Eandi CM, Ventre L, Rigault de la Longrais RC, Grignolo FM. Photodynamic therapy for chronic central serous chorioretinopathy. Retina 2003;23(6):752‐63. - PubMed
Cassin 2006
-
- Cassin B. Dictionary of Eye Terminology. 5th Edition. Gainesville, Florida: Triad Publishing Company, 2006.
Castro‐Correia 1992
-
- Castro‐Correia J, Countinho MF, Rosas V, Maia J. Long‐term follow‐up of central serous retinopathy in 150 patients. Documenta Ophthalmologica 1992;81(4):379‐86. - PubMed
Chaimani 2013
Chan 2003
-
- Chan WM, Lam DS, Lai TY, Yuen KS, Liu DT, Chan CK, et al. Treatment of choroidal neovascularization in central serous chorioretinopathy by photodynamic therapy with verteporfin. American Journal of Opthalmology 2003;136(5):836‐45. - PubMed
Chen 2008
-
- Chen SN, Hwang JF, Tseng LF, Lin CJ. Subthreshold diode micropulse photocoagulation for the treatment of chronic central serous chorioretinopathy with juxtafoveal leakage. Ophthalmology 2008;115(12):2229‐34. - PubMed
Chou 2000
-
- Chou SC, Lin JD. Long‐term effects of ketoconazole in the treatment of residual or recurrent Cushing's disease. Endocrine Journal 2000;47(4):401‐6. - PubMed
Chung 2013
Clark 2008
Cotticelli 2006
-
- Cotticelli L, Borrelli M, D'Alessio AC, Menzione M, Villani A, Piccolo G, et al. Central serous chorioretinopathy and Helicobacter pylori. European Journal of Ophthalmology 2006;16(2):274‐8. - PubMed
Covidence
-
- Covidence. www.covidence.org (accessed 20 April 2015).
Cox 1988
-
- Cox SN, Hay E, Bird AC. Treatment of chronic macular edema with acetazolamide. JAMA Ophthalmology 1988;106(9):1190‐5. - PubMed
FDA 2015
-
- Drugs@FDA. U.S. Food and Drug Administration. https://www.accessdata.fda.gov/scripts/cder/drugsatfda/ (accessed 25 August 2015).
Forooghian 2011
Gass 1967
-
- Gass JD. Pathogenesis of disciform detachment of the neuroepithelium. American Journal of Ophthalmology 1967;63(3):S1‐139. - PubMed
Giusti 2004
-
- Giusti C. Association of Helicobacter pylori with central serous chorioretinopathy: hypotheses regarding pathogenesis. Medical Hypotheses 2004;63(3):524‐7. - PubMed
Glanville 2006
Grieshaber 2007
Guengerich 1999
-
- Guengerich FP. Cytochrome P‐450 3A4: regulation and role in drug metabolism. Annual Review of Pharmacology and Toxicology 1999;39:1‐17. - PubMed
Guyatt 2011
-
- Guyatt GH, Oxman AD, Schunemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64:380‐2. - PubMed
Haimovici 2004
-
- Haimovici R, Koh S, Gagnon DR, Lehrfeld T, Wellik S, Central Serous Chorioretinopathy Case‐Control Study Group. Risk factors for central serous chorioretinopathy: a case‐control study. Ophthalmology 2004;111(2):244‐9. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2012
Hofstetter 2000
-
- Hofstetter WH, Griffin JR, Berman MS, Everson RW. Visual Science and Related Clinical Terms. 5th Edition. Woburn: Butterworth‐Heinemann, 2000.
Iijima 1999
-
- Iijima H, Iida T, Murayama K, Imai M, Gohdo T. Plasminogen activator inhibitor 1 in central serous chorioretinopathy. American Journal of Ophthalmology 1999;127(4):477‐8. - PubMed
Jampol 2002
-
- Jampol LM, Weinreb R, Yannuzzi L. Involvement of corticosteroids and catecholamines in the pathogenesis of central serous chorioretinopathy: a rationale for new treatment strategies. Ophthalmology 2002;109(10):1765‐6. - PubMed
Kim 2009
-
- Kim SW, Oh J, Oh IK, Huh K. Retinal pigment epithelial tear after half fluence PDT for serous pigment epithelial detachment in central serous chorioretinopathy. Ophthalmic Surgery, Lasers and Imaging Retina 2009;40(3):300‐3. - PubMed
Kitzmann 2008
-
- Kitzmann AS, Pulido JS, Diehl NN, Hodge DO, Burke JP. The incidence of central serous chorioretinopathy in Olmsted County, Minnesota, 1980‐2002. Ophthalmology 2008;115(1):169‐73. - PubMed
Klein 1974
-
- Klein ML, Buskirk EM, Friedman E, Gragoudas E, Chandra S. Experience with nontreatment of central serous choroidopathy. Archives of Ophthalmology 1974;91(4):247‐50. - PubMed
Lai 2006
Lim 2014
-
- Lim JI, Glassman AR, Aiello LP, Chakravarthy U, Flaxel CJ, Spaide RF. Collaborative retrospective macula society study of photodynamic therapy for chronic central serous chorioretinopathy. Ophthalmology 2014;121:1073‐8. - PubMed
Loo 2002
-
- Loo RH. Factors associated with reduced visual acuity during long‐term follow‐up of patients with idiopathic central serous chorioretinopathy. Retina 2002;22(1):19‐24. - PubMed
Ma 2014
-
- Ma J, Meng N, Xu X, Zhou F, Qu Y. System review and meta‐analysis on photodynamic therapy in central serous chorioretinopathy. Acta Ophthalmologica 2014;92:e594‐601. - PubMed
Maalej 2014
-
- Maalej A, Khallouli A, Wathek C, Rannen R, Gabsi S. Central serous chorioretinopathy: clinical‐anatomic correlations. Journal Français d'Ophtalmologie 2014;37(10):787‐95. - PubMed
Maruko 2010
-
- Maruko I, Iida T, Sugano Y, Ojima A, Ogasawara M, Spaide RF. Subfoveal choroidal thickness after treatment of central serous chorioretinopathy. Ophthalmology 2010;117(9):1792‐9. - PubMed
Meyerle 2007
-
- Meyerle CB, Freund KB, Bhatnagar P, Shah V, Yannuzzi LA. Ketoconazole in the treatment of chronic idiopathic central serous chorioretinopathy. Retina 2007;27(7):943‐6. - PubMed
Mitsui 1969
-
- Mitsui Y, Matsubara M, Kanagawa M. Xenon light‐exposure as a treatment of central serous retinopathy (a preliminary report). Nippon Ganka Kiyo 1969;20(3):291‐4. - PubMed
Montero 2005
Mudvari 2007
-
- Mudvari SS, Goff MJ, Fu AD, McDonald HR, Johnson RN, Ai E. The natural history of pigment epithelial detachment associated with central serous chorioretinopathy. Retina 2007;27(9):1168‐73. - PubMed
Nicholson 2013
Prunte 1996
-
- Prunte C, Flammer J. Choriodal capillary and venous congestion in central serous chorioretinopathy. American Journal of Ophthalmology 1996;121(1):26‐34. - PubMed
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ 2014;349:g5630. - PubMed
Quin 2013
-
- Quin G, Liew G, Ho I, Gillies M, Fraser‐Bell S. Diagnosis and interventions for central serous chorioretinopathy: review and update. Clinical and Experimental Ophthalmology 2013;41(2):187‐200. - PubMed
Reibaldi 2010
-
- Reibaldi M, Cardascia N, Longo A, Furino C, Avitabile T, Faro S, et al. Standard‐fluence versus low‐fluence photodynamic therapy in chronic central serous chorioretinopathy: a nonrandomized clinical trial. American Journal of Ophthalmology 2010;149(2):307‐15. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricci 2004
-
- Ricci F, Missiroli F, Cerulli L. Indocyanine green dye‐enhanced micropulsed diode laser: a novel approach to subthreshold RPE treatment in a case of central serous chorioretinopathy. European Journal of Ophthalmology 2004;14(1):74‐82. - PubMed
Schlötzer‐Schrehardt 2002
-
- Schlötzer‐Schrehardt U, Viestenz A, Naumann GO, Laqua H, Michels S, Schmidt‐Erfurth U. Dose‐related structural effects of photodynamic therapy on choroidal and retinal structures of human eyes. Graefe's Archive for Clinical and Experimental Ophthalmology 2002;240(9):748‐57. - PubMed
Schmidt‐Erfurth 2002
-
- Schmidt‐Erfurth U, Michels S, Barbazetto I, Laqua H. Photodynamic effects on choroidal neovascularization and physiological choroid. Investigative Ophthalmology and Visual Science 2002;43(3):830‐41. - PubMed
Shuler 2006
-
- Shuler RK, Mruthyunjaya P. Diagnosing and managing central serous chorioretinopathy. EyeNet Magazine, February 2006. Available at www.aao.org/publications/eyenet/200602/pearls.cfm (accessed 13 March 2015).
Sivaprasad 2010
-
- Sivaprasad S, Elagouz M, McHugh D, Shona O, Dorin G. Micropulsed diode laser therapy: evolution and clinical applications. Survey of Ophthalmology 2010;55(6):516‐30. - PubMed
Sterne 2011
-
- Sterne JAC, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Systematic Review Data Repository
-
- Systematic Review Data Repository (SRDR). srdr.ahrq.gov/ (accessed 20 April 2015).
Wang 2008
-
- Wang M, Munch IC, Hasler PW, Prunte C, Larsen M. Central serous chorioretinopathy. Acta Opthalmologica 2008;86(2):126‐45. - PubMed
White 2009
-
- White IR. Multivariate random‐effects meta‐analysis. Stata Journal 2009;9:40‐56.
White 2011
-
- White IR. Multivariate random‐effects meta‐regression: updates to mvmeta. Stata Journal 2011;11:255‐70.
White 2012
Winquist 1995
-
- Winquist EW, Laskey J, Crump M, Khamsi F, Shepherd FA. Ketoconazole in the management of paraneoplastic Cushing's syndrome secondary to ectopic adrenocorticotropin production. Journal of Clinical Oncology 1995;13(1):157‐64. - PubMed
Yannuzzi 1987
-
- Yannuzzi LA. Type‐A behavior and central serous chorioretinopathy. Retina 1987;7(2):111‐31. - PubMed
Yanoff 2013
-
- Yanoff M, Duker J. Ophthalmology. 4th Edition. Saunders, 2013.
References to other published versions of this review
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

